Editorial Focus
Jeffrey E. Gotts and Michael A. Matthay
Mesenchymal stem cells and the stem cell niche: a new chapter
Am J Physiol Lung Cell Mol Physiol June 1, 2012 302:L1147-L1149

BONE MARROW-DERIVED mesenchymal stem/stromal cells (MSCs) are self-renewing multipotent cells with therapeutic effects in diverse models of tissue injury. In the rodent lung, MSCs reduce collagen deposition in the bleomycin model of pulmonary fibrosis and reduce lung injury and improve survival following intrapulmonary delivery of endotoxin or Escherichia coli and following severe gram-negative peritonitis. In the hyperoxia model of bronchopulmonary dysplasia, exposure to high concentrations of oxygen during early postnatal life in rats and mice causes simplification of alveolar and lung capillary structure and reduced pulmonary capillary surface area, leading to pulmonary hypertension. Two groups reported simultaneously in 2009 that MSCs given by airway to rats or by blood to mice during prolonged hyperoxia in early postnatal life prevented arrested alveolar growth. However, engraftment of MSCs during hyperoxia and in other models has not accounted for the therapeutic effects, thus prompting a search for other mechanisms. MSCs are potent immunomodulators, suppressing several functions of lymphocytes, natural killer cells, and monocytes, and reduce inflammatory cell lung infiltrates and cytokines during sepsis and acute lung injury. In addition, MSCs have direct antibacterial effects, secrete epithelial growth factors, and can rescue epithelial cellular bioenergetics with mitochondrial transfer.
Original Articles
■ β-Adrenergic agonists differentially regulate highly selective and nonselective epithelial sodium channels to promote alveolar fluid clearance in vivo
Charles A. Downs, Lisa H. Kriener, Ling Yu, Douglas C. Eaton, Lucky Jain, and My N. Helms
Am J Physiol Lung Cell Mol Physiol June 1, 2012 302:L1167-L1178
ENaCの活性化は,β1受容体でもβ2受容体刺激でも生じ,肺の水クリアランスを改善する。
デノパミン β1-stimulation
タブタリン β2-stimulation
β-Adrenergic receptors (β-AR) increase epithelial sodium channel (ENaC) activity to promote lung fluid clearance. However, the effect of selective β-AR agonist on highly selective cation (HSC) channels or nonselective cation (NSC) channels in alveolar type 1 (T1) and type 2 (T2) cells is unknown. We hypothesized that stimulation with β(1)-AR agonist (denopamine) or β(2)-AR agonist (terbutaline) would increase HSC and/or NSC channel activity in alveolar epithelial cells. We performed single-channel measurements from T1 and T2 cells accessed from rat lung slices. Terbutaline (20 μM) increased HSC ENaC activity (open probability, NP(o)) in T1 (from 0.96 ± 0.61 to 1.25 ± 0.71, n = 5, P
■ The p110δ subunit of PI3K regulates bone marrow-derived eosinophil trafficking and airway eosinophilia in allergen-challenged mice
Bit Na Kang, Sung Gil Ha, Xiao Na Ge, M. Reza Hosseinkhani, Nooshin S. Bahaie, Yana Greenberg, Malcolm N. Blumenthal, Kamal D. Puri, Savita P. Rao, and P. Sriramarao
Am J Physiol Lung Cell Mol Physiol June 1, 2012 302:L1179-L1191
好酸球のリクルートメントとトラフィッキング
CD11b・・MAC1
CD49・・α4
CRA:cockroach antigenでのマウスの気道過敏性に関するin vivo研究も併設
Trafficking and recruitment of eosinophils during allergic airway inflammation is mediated by the phosphatidylinositol 3-kinase (PI3K) family of signaling molecules. The role played by the p110δ subunit of PI3K (PI3K p110δ) in regulating eosinophil trafficking and recruitment was investigated using a selective pharmacological inhibitor (IC87114). Treatment with the PI3K p110δ inhibitor significantly reduced murine bone marrow-derived eosinophil (BM-Eos) adhesion to VCAM-1 as well as ICAM-1 and inhibited activation-induced changes in cell morphology associated with reduced Mac-1 expression and aberrant cell surface localization/distribution of Mac-1 and α4. Infused BM-Eos demonstrated significantly decreased rolling and adhesion in inflamed cremaster muscle microvessels of mice treated with IC87114 compared with vehicle-treated mice. Furthermore, inhibition of PI3K p110δ significantly attenuated eotaxin-1-induced BM-Eos migration and prevented eotaxin-1-induced changes in the cytoskeleton and cell morphology. Knockdown of PI3K p110δ with siRNA in BM-Eos resulted in reduced rolling, adhesion, and migration, as well as inhibition of activation-induced changes in cell morphology, validating its role in regulating trafficking and migration. Finally, in a mouse model of cockroach antigen-induced allergic airway inflammation, oral administration of the PI3K p110δ inhibitor significantly inhibited airway eosinophil recruitment, resulting in attenuation of airway hyperresponsiveness in response to methacholine, reduced mucus secretion, and expression of proinflammatory molecules (found in inflammatory zone-1 and intelectin-1). Overall, these findings indicate the important role played by PI3K p110δ in mediating BM-Eos trafficking and migration by regulating adhesion molecule expression and localization/distribution as well as promoting changes in cell morphology that favor recruitment during inflammation.
■ RAGE35
RAGE signaling by alveolar macrophages influences tobacco smoke-induced inflammation
Adam B. Robinson, KacyAnn D. Johnson, Brock G. Bennion, and Paul R. Reynolds
Am J Physiol Lung Cell Mol Physiol June 1, 2012 302:L1192-L1199
CSE・・タバコの煙暴露モデル:肺胞マクロファージのRAGEが増加する
cigarette smoke extractはRAGEのリガンドか,RAGEリガンドを産生する可能性がある。
RAGE→RAS→P38MAPK ?
RAGE-KOモデルの使用
Receptors for advanced glycation end-products (RAGE) are multiligand cell surface receptors of the immunoglobin family expressed by epithelium and macrophages, and expression increases following exposure to cigarette smoke extract (CSE). The present study sought to characterize the proinflammatory contributions of RAGE expressed by alveolar macrophages (AMs) following CSE exposure. Acute exposure of mice to CSE via nasal instillation revealed diminished bronchoalveolar lavage (BAL) cellularity and fewer AMs in RAGE knockout (KO) mice compared with controls. Primary AMs were obtained from BAL, exposed to CSE in vitro, and analyzed. CSE significantly increased RAGE expression by wild-type AMs. Employing ELISAs, wild-type AMs exposed to CSE had increased levels of active Ras, a small GTPase that perpetuates proinflammatory signaling. Conversely, RAGE KO AMs had less Ras activation compared with wild-type AMs after exposure to CSE. In RAGE KO AMs, assessment of p38 MAPK and NF-κB, important intracellular signaling intermediates induced during an inflammatory response, revealed that CSE-induced inflammation may occur in part via RAGE signaling. Lastly, quantitative RT-PCR revealed that the expression of proinflammatory cytokines including TNF-α and IL-1β were detectably decreased in RAGE KO AMs exposed to CSE compared with CSE-exposed wild-type AMs. These results reveal that primary AMs orchestrate CSE-induced inflammation, at least in part, via RAGE-mediated mechanisms.
■ Platelets induce endothelial tissue factor expression in a mouse model of acid-induced lung injury
Memet T. Emin, Li Sun, Alice Huertas, Shonit Das, Jahar Bhattacharya, and Sunita Bhattacharya
Am J Physiol Lung Cell Mol Physiol June 1, 2012 302:L1209-L1220
Dept. of Pediatrics, Columbia Univ., 630 W. 168th St., BB 8-812, New York, NY 10032. sb80@columbia.edu.
Although the lung expresses procoagulant proteins under inflammatory conditions, underlying mechanisms remain unclear. Here, we addressed lung endothelial expression of tissue factor (TF), which initiates the coagulation cascade and expression of which signifies development of a procoagulant phenotype in the vasculature. To establish the model of acid-induced acute lung injury (ALI), we intranasally instilled anesthetized mice with saline or acid. Then 2 h later, we isolated pulmonary vascular cells for flow cytometry and confocal microscopy to detect the leukocyte antigen, CD45 and the endothelial markers VE-cadherin and von Willebrand factor (vWf). Acid increased both the number of vWf-expressing cells as well as TF and P-selectin expressions on these cells. All of these effects were markedly inhibited by treating mice with antiplatelet serum, suggesting the involvement of platelets. The increased expressions of TF, vWf, and P-selectin in response to acid also occurred in platelets. Moreover, the effects were replicated in endothelial cells derived from isolated, blood-perfused lungs. However, the effect was inhibited completely in lungs perfused with platelet-depleted and, to a lesser extent, with leukocyte-depleted blood. Acid injury increased endothelial expressions of the platelet proteins, CD41 and CD42b, providing evidence that platelet proteins were transferred to the vascular surface. Reactive oxygen species (ROS) were implicated in these responses, in that the endothelial and platelet protein expressions were inhibited. We conclude that acid-induced ALI causes NOX2-mediated ROS generation that activates platelets, which then generate a procoagulant endothelial surface.
■ Interaction with CREB binding protein modulates the activities of Nrf2 and NF-κB in cystic fibrosis airway epithelial cells
Assem G. Ziady, Andrew Sokolow, Samuel Shank, Deborah Corey, Ross Myers, Scott Plafker, and Thomas J. Kelley
Am J Physiol Lung Cell Mol Physiol June 1, 2012 302:L1221-L1231
cAMP→CREB→NRF2活性
cAMP活性によるNRF2活性vsNF-κB活性の調節作用
NRF2はKeap1で制御されており,25個のシステインのうち,Cys151,Cys273,Cys288などが酸化修飾を受け,NRF2のユビキチン化が減少し,NRF2活性が持続することにより,抗酸化物質の転写が亢進する。
Cystic fibrosis (CF) is characterized by inflammatory lung disease that significantly contributes to morbidity and mortality. Airway epithelial cells play a role in the inflammatory signaling in CF and have been reported to exhibit a number of dysfunctions in signaling cascades that modulate inflammation. Previously, we reported that the activity of nuclear factor erythroid-derived-like 2 (Nrf2), a transcription factor that regulates antioxidant and cytoprotective protein expression, is diminished in CF epithelia (7). In this report, we examined the mechanism of Nrf2 dysregulation in vitro in human airway epithelial cell lines and primary cells and in vivo in nasal epithelia excised from ΔF508 CF mutant mice. We found that cAMP-mediated signaling markedly reduces Nrf2 activity in CF vs. non-CF cells. Rp-cAMPS, a cAMP competitor, significantly corrected Nrf2 activity in CF cells, predominantly by increasing the nuclear accumulation of the transcription factor. Furthermore, we found that Rp-cAMPS significantly decreased NF-κB activation following inflammatory stimulation of CF cells. Further investigation revealed that Nrf2 and NF-κB compete for the transcriptional coactivator cAMP responsive element-binding protein (CREB) binding protein (CBP) and that Rp-cAMPS shifts CBP association in favor of Nrf2. Thus our findings provide a link between feedback to CF transmembrane regulator dysfunction and dysregulation of an inflammatory signaling pathway that modulates the coordinated activities of Nrf2 and NF-κB. Furthermore, our studies suggest that strategies that shift CBP association away from NF-κB and toward Nrf2 could have potential therapeutic efficacy for reducing inflammation in patients with CF.
最新の画像[もっと見る]
-
 お知らせ 世界敗血症デー 2024 KYOTO
3週間前
お知らせ 世界敗血症デー 2024 KYOTO
3週間前
-
 セミナーのお知らせ 多発外傷カンファレンス with デューク大学
1ヶ月前
セミナーのお知らせ 多発外傷カンファレンス with デューク大学
1ヶ月前
-
 執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
2ヶ月前
執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
2ヶ月前
-
 執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
2ヶ月前
執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
2ヶ月前
-
 執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
2ヶ月前
執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
2ヶ月前
-
 救急・集中治療 人食いバクテリアの診断と治療
7ヶ月前
救急・集中治療 人食いバクテリアの診断と治療
7ヶ月前
-
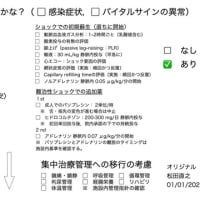 救急・集中治療 人食いバクテリアの診断と治療
7ヶ月前
救急・集中治療 人食いバクテリアの診断と治療
7ヶ月前
-
 謹賀新年 2024年
7ヶ月前
謹賀新年 2024年
7ヶ月前
-
 謹賀新年 2024年
7ヶ月前
謹賀新年 2024年
7ヶ月前
-
 【WEBセミナー】Global Sepsis Allianceからのお知らせ
8ヶ月前
【WEBセミナー】Global Sepsis Allianceからのお知らせ
8ヶ月前
「ジャーナルクラブ 松田直之指導」カテゴリの最新記事
 ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年12月号 Sonic Hed...
ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年12月号 Sonic Hed... 2012年12月13日 基盤ジャーナルクラブ 心血管基礎研究関連
2012年12月13日 基盤ジャーナルクラブ 心血管基礎研究関連 文献紹介 Hypoglycemia and Risk of Death in Critically Ill Patients N Engl J...
文献紹介 Hypoglycemia and Risk of Death in Critically Ill Patients N Engl J... ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年11月号
ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年11月号 ジャーナルクラブ Circulation Res 2012, September 14 & 28
ジャーナルクラブ Circulation Res 2012, September 14 & 28 ジャーナルクラブ British J Pharmacol 2012. October Volume 167, Issue 3
ジャーナルクラブ British J Pharmacol 2012. October Volume 167, Issue 3 ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年9月号
ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年9月号 ジャーナルクラブ Circulation Research 2012. August
ジャーナルクラブ Circulation Research 2012. August ジャーナルクラブ British Journal of Pharmacology 2012. September
ジャーナルクラブ British Journal of Pharmacology 2012. September ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年7月号
ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年7月号

















