総説 血管内皮細胞のTRPA1について
You have free access to this contentTRPA1 channels in the vasculature (pages 13–22)
Scott Earley
Article first published online: 3 AUG 2012 | DOI: 10.1111
Abstruct
This review is focused on the role of the ankyrin (A) transient receptor potential (TRP) channel TRPA1 in vascular regulation. TRPA1 is activated by environmental irritants, pungent compounds found in foods such as garlic, mustard and cinnamon, as well as metabolites produced during oxidative stress. The structure of the channel is distinguished by the ∼14–19 ankyrin repeat (AR) domains present in the intracellular amino terminus. TRPA1 has a large unitary conductance (98 pS) and slight selectivity for Ca2+ versus Na+ ions (PCa/PNa ≈ 7.9). TRPA1 is involved in numerous important physiological processes, including nociception, mechanotransduction, and thermal and oxygen sensing. TRPA1 agonists cause arterial dilation through two distinctive pathways. TRPA1 channels present in perivascular nerves mediate vasodilatation of peripheral arteries in response to chemical agonists through a mechanism requiring release of calcitonin gene-related peptide. In the cerebral circulation, TRPA1 channels are present in the endothelium, concentrated within myoendothelial junction sites. Activation of TRPA1 channels in this vascular bed causes endothelium-dependent smooth muscle cell hyperpolarization and vasodilatation that requires the activity of small and intermediate conductance Ca2+-activated K+ channels. Systemic administration of TRPA1 agonists causes transient depressor responses, followed by sustained increases in heart rate and blood pressure that may result from elevated sympathetic nervous activity. These findings indicate that TRPA1 activity influences vascular function, but the precise role and significance of the channel in the cardiovascular system remains to be determined.
Transient Receptor Potential superfamily について
The mammalian transient receptor potential (TRP) superfamily of cation channels comprises 28 members assigned to six subfamilies based on sequence homology. The ankyrin (A) subfamily is the smallest and is composed of only a single member, TRPA1 (originally designated as ANKTM1). Despite being one of the last TRP channels to be discovered (Story et al., 2003), TRPA1 has garnered a great deal of recent attention. The essential properties and structure of TRPA1 have been evolutionarily conserved for more than 500 million years (Kang et al., 2010). The channel likely evolved as a sensor of electrophilic toxicity (Kang et al., 2010) before divergent specialized functions developed in different species (Story et al., 2003; Rosenzweig et al., 2005; Cordero-Morales et al., 2011; Geng et al., 2011). TRPA1, like all TRP channels, is expressed as six-transmembrane domain polypeptide subunits, a motif common to many types of ion channels. Functional TRPA1 channels are formed from four of these subunits. Assembled TRPA1 channels are thought to have a homomeric structure (composed of four identical subunits), as there is currently no evidence that heteromultimeric channels involving other TRP channel subunits can form. The channel is distinguished structurally by, and named for, the ∼14–19 ankyrin repeat (AR) domains forming a portion of the protein's intracellular N-terminus. In general, AR domains mediate protein–protein interactions and provide mechanical elasticity (Sedgwick and Smerdon, 1999), although a recent study suggests that particular TRPA1 AR domains can regulate agonist- and heat-induced channel activity (Cordero-Morales et al., 2011). TRPA1 was originally described as a non-selective cation channel that is equally permeable to Na+ versus Ca2+ ions (PCa/PNa reported as 0.84–3.28) (Story et al., 2003; Wang et al., 2008), although Karashima et al. found that during agonist stimulation, PCa/PNa = 7.91 ± 0.60 and the fractional Ca2+ current under these conditions is 17.9–22.3% (Karashima et al., 2010). The unitary conductance of the channel is large (98 pS, when physiological ionic gradients are maintained) (Nagata et al., 2005), indicating that TRPA1 channels can support consequential levels of Ca2+ influx. Predictably, TRPA1 has been shown to influence a broad range of physiological processes that involve Ca2+-dependent signalling pathways, including nociception, mechanotransduction, thermal and oxygen sensing, and responses to environmental irritants and pungent compounds. This manuscript focuses on the role of TRPA1 channels in vascular regulation. The relevant pharmacology is discussed, and studies investigating the consequences of TRPA1 activity on local and integrative control of the vasculature are reviewed.
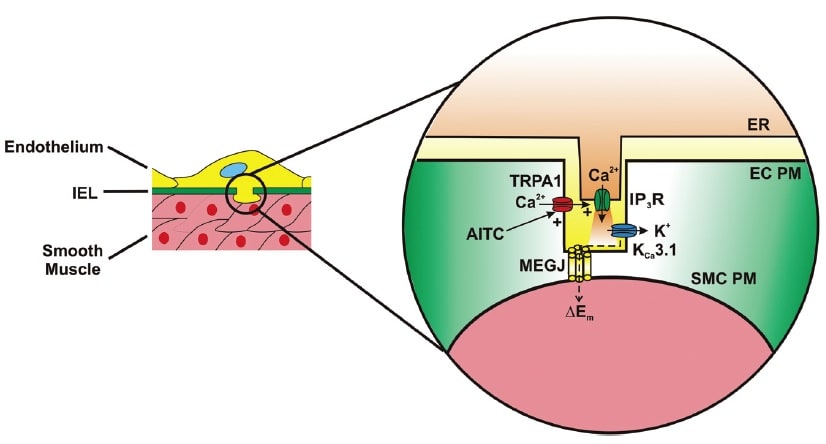
Activation of TRPA1 in cerebral arteries causes endothelium-dependent vasodilation. Allyl isothiocyanate (AITC) activates Ca2+ influx via TRPA1 channels present in myoendothelial junctions in cerebral arteries. TRPA1-mediated Ca2+ influx stimulates Ca2+ release from the endoplasmic reticulum (ER) via inositol trisphosphate receptors (IP3R). The resulting Ca2+ signal (i.e. Ca2+ pulsar) stimulates proximal intermediate conductance Ca2+-activated K+ channels (KCa3.1), resulting in hyperpolarization of the endothelial cell plasma membrane (EC PM). The change in membrane potential (DEm) is conducted via myoendothelial gap junctions (MEGJs) to hyperpolarize the vascular smooth muscle cell plasma membrane (SMCPM), resulting in myocyte relaxation.

Activation of TRPA1 channels in sensory nerves causes arterial dilation. Allyl isothiocyanate (AITC), allicin, cinnamaldehyde (CA) and 4-oxo-2-nonenal (4-ONE) activate Ca2+ influx via TRPA1 channels in sensory nerves, causing release of calcitonin gene-related peptide (CGRP) from perivascular terminals. CGRP binds to its G protein-coupled receptor (GPCR) on the plasma membrane of vascular smooth muscle cells (SMCs) to cause membrane hyperpolarization and myocyte relaxation.
RESEARCH PAPERS
1. メラノコルチン受容体MC1とMC3を介した炎症の保護作用 <炎症のタイムコースが大切>
Chondroprotective and anti-inflammatory role of melanocortin peptides in TNF-α activated human C-20/A4 chondrocytes (pages 67–79)
Magdalena K Kaneva, Mark JP Kerrigan, Paolo Grieco, G Paul Curley, Ian C Locke and Stephen J Getting
Article first published online: 3 AUG 2012 | DOI: 10.1111/j.1476-5381.
BACKGROUND AND PURPOSE
Melanocortin MC1 and MC3 receptors, mediate the anti-inflammatory effects of melanocortin peptides. Targeting these receptors could therefore lead to development of novel anti-inflammatory therapeutic agents. We investigated the expression of MC1 and MC3 receptors on chondrocytes and the role of α-melanocyte-stimulating hormone (α-MSH) and the selective MC3 receptor agonist, [DTRP8]-γ-MSH, in modulating production of inflammatory cytokines, tissue-destructive proteins and induction of apoptotic pathway(s) in the human chondrocytic C-20/A4 cells.
EXPERIMENTAL APPROACH
Effects of α-MSH, [DTRP8]-γ-MSH alone or in the presence of the MC3/4 receptor antagonist, SHU9119, on TNF-α induced release of pro-inflammatory cytokines, MMPs, apoptotic pathway(s) and cell death in C-20/A4 chondrocytes were investigated, along with their effect on the release of the anti-inflammatory cytokine IL-10.
KEY RESULTS
C-20/A4 chondrocytes expressed functionally active MC1,3 receptors. α-MSH and [DTRP8]-γ-MSH treatment, for 30 min before TNF-α stimulation, provided a time-and-bell-shaped concentration-dependent decrease in pro-inflammatory cytokines (IL-1β, IL-6 and IL-8) release and increased release of the chondroprotective and anti-inflammatory cytokine, IL-10, whilst decreasing expression of MMP1, MMP3, MMP13 genes.α-MSH and [DTRP8]-γ-MSH treatment also inhibited TNF-α-induced caspase-3/7 activation and chondrocyte death. The effects of [DTRP8]-γ-MSH, but not α-MSH, were abolished by the MC3/4 receptor antagonist, SHU9119.
CONCLUSION AND IMPLICATIONS
Activation of MC1/MC3 receptors in C-20/A4 chondrocytes down-regulated production of pro-inflammatory cytokines and cartilage-destroying proteinases, inhibited initiation of apoptotic pathways and promoted release of chondroprotective and anti-inflammatory cytokines. Developing small molecule agonists to MC1/MC3 receptors could be a viable approach for developing chondroprotective and anti-inflammatory therapies in rheumatoid and osteoarthritis.
2. PAR1 inhibitor Q94/Q109について
Modulation of PAR1 signalling by benzimidazole compounds (pages 80–94)
S Asteriti, S Daniele, F Porchia, MT Dell' Anno, A Fazzini, I Pugliesi, ML Trincavelli, S Taliani, C Martini, MR Mazzoni and A Gilchrist
Article first published online: 3 AUG 2012 | DOI: 10.1111/j.1476-5381.
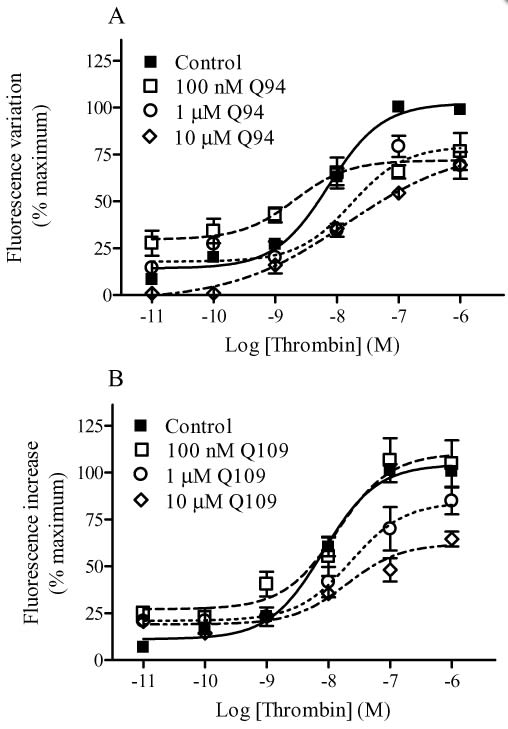
Modulation of thrombin-induced intracellular Ca2+ mobilization by Q94 (A) and Q109 (B). Intracellular Ca2+ mobilization was measured using Fluo 3-AM-loaded HMECs-1 as described in Methods. Basal [Ca2+]i was 240.1 ± 45.3 nM (n = 4). The benzimidazole compounds were added 15 min before the addition of thrombin. The concentration–response curves were performed using seven different concentrations of the enzyme in the presence and absence of fixed concentrations of PAR1 modulators. Data are reported as % of maximal RF and are the means ± SEM of three (A) and four (B) independent experiments, each performed in triplicate.
研究内容:
BACKGROUND AND PURPOSE
Recently, a small molecule (Q94) was reported to selectively block PAR1/Gαq interaction and signalling. Here, we describe the pharmacological properties of Q94 and two analogues that share its benzimidazole scaffold (Q109, Q89). Q109 presents a modest variation from Q94 in the substituent group at the 2-position, while Q89 has quite different groups at the 1- and 2-positions.
EXPERIMENTAL APPROACH
Using human microvascular endothelial cells, we examined intracellular Ca2+ mobilization and inositol 1,4,5-trisphosphate accumulation as well as isoprenaline- or forskolin-stimulated cAMP production in response to thrombin.
KEY RESULTS
Q89 (10 µM) produced a leftward shift in the thrombin-mediated intracellular Ca2+ mobilization concentration–response curve while having no effect on the Emax. Both Q94 (10 µM) and Q109 (10 µM) reduced intracellular Ca2+ mobilization, leading to a decrease in Emax and an increase in EC50 values. Experiments utilizing receptor-specific activating peptides confirmed that Q94 and Q109 were selective for PAR1 as they did not alter the Ca2+ response mediated by a PAR2 activating peptide. Consistent with our Ca2+ results, micromolar concentrations of either Q94 or Q109 significantly reduced thrombin-induced inositol 1,4,5-trisphosphate production. Neither Q94 nor Q109 diminished the inhibitory effects of thrombin on cAMP production, indicating they inhibit signalling selectively through the Gq pathway. Our results also suggest the 1,2-disubstituted benzimidazole derivatives act as ‘allosteric agonists’ of PAR1.
CONCLUSIONS AND IMPLICATIONS
The Q94 and Q109 benzimidazole derivatives represent a novel scaffold for the development of new PAR1 inhibitors and provide a starting point to develop dual signalling pathway-selective positive/negative modulators of PAR1.
Introductionの記載
Protease-activated receptors (PARs) are a family of four GPCRs (PAR1, PAR2, PAR3, and PAR4; receptor nomenclature follows Alexander et al., 2011) characterized by a unique mechanism of activation. PARs are activated enzymatically through proteolysis of the receptor by enzymes of the serine protease family (Macfarlane et al., 2001). The proteolytic cleavage occurs at specific sites within their N-terminal region, thereby exposing novel N-termini, and the ‘tethered ligand’ then folds back onto the extracellular loop II of the receptor, resulting in activation. PAR1, PAR3, and PAR4 are preferentially cleaved by thrombin; whereas PAR2 is mainly a substrate for trypsin, and mast cell tryptase (Coughlin, 2001; Macfarlane et al., 2001; Hollenberg and Compton, 2002). In addition to proteolytic cleavage, most PARs can be activated by synthetic peptides corresponding to the tethered ligand (TL) sequence (Ramachandran and Hollenberg, 2008).
PARs are expressed in many cell types and different organ systems. For example, PAR1, PAR2, PAR3 and PAR4 are all expressed on human endothelial cells (Ramachandran and Hollenberg, 2008); although PAR4 expression may be localized to the endothelium of specific vascular areas (O'Brien et al., 2000; Fujiwara et al., 2005; Hirano et al., 2007). Upon activation, PAR1 exerts its effects on endothelium by activating multiple G-proteins, including Gi/o, Gq/11 and G12/13, leading to modulation of numerous downstream signalling pathways (Barr et al., 1997; Vanhauwe et al., 2002; Ramachandran and Hollenberg, 2008). While PAR1 expression is widely distributed among cells and tissues, PAR2 expression is more limited, and studies indicate that signalling occurs via Gq/11, Gi/o (Nystedt et al., 1995; Macfarlane et al., 2001) and perhaps G12/13 (Ramachandran et al., 2009).
PAR activation plays a key role in many physiological and pathophysiological events involving different organ systems (Ramachandran and Hollenberg, 2008). For example, in the cardiovascular and circulatory systems, activation of PAR1 and to a lesser extent PAR4 on human platelets is sufficient to trigger aggregation (Kahn et al., 1999), while activation of human endothelial PAR1 and PAR2 causes vascular relaxation (Hamilton et al., 2001; 2002; Robin et al., 2003). Indeed, PAR antagonists might prove useful therapeutically for the treatment of several diseases, including thrombosis and atherosclerosis.
Several peptide, peptidomimetic and non-peptide PAR1 antagonists are currently available for experimental studies; and a number of synthetic small molecules are being evaluated for pharmaceutical use in humans (reviewed by Chackalamannil, 2006). In addition, alternative approaches to inhibit PAR1 signalling, such as transfection of endothelial cells with minigene vectors expressing Gα carboxyl (C)-terminal peptides (Gilchrist et al., 2001), or the use of membrane-permeable peptides termed ‘pepducins’ derived from the sequence of the third intracellular loop of PAR1 (Covic et al., 2002a,b), have been presented. Deng et al. (2008) reported the use of a small molecule, Q94, which selectively blocks the interaction between PAR1 and Gαq, to investigate thrombin mediated signalling in mouse lung fibroblasts. Q94 was originally identified during an elisa screen for competition of a high-affinity peptide mimicking the C-terminus of Gαq using a compound library (Deng et al., 2008), and the compound may act as a negative allosteric modulator of PAR1 rather than an orthosteric antagonist. Although Q94 has not been extensively investigated, it represents the first compound to show selective modulation of PAR1/G-protein interactions and thus serve as a biased inhibitor of thrombin-mediated Gq pathway signalling events.
In the present study, we examined the pharmacological properties of Q94 and two analogues, Q109 and Q89, using human microvascular endothelial cells. The three small molecules (Q94, Q109, Q89) all share a benzimidazole scaffold and present either a modest variation in the substituent group at the 2-position (Q109 vs. Q94) or quite different groups at the 1- and 2-positions (Q89 vs. Q94). Whereas micromolar concentrations of Q94 or Q109 resulted in a 30–50% reduction of thrombin's maximal effect (Emax) on intracellular Ca2+ mobilization in combination with a two- to threefold increase of thrombin's EC50 value, a micromolar concentration of Q89 produced a shift to the left in thrombin's concentration–response curve and had no effect on thrombin Emax. Similar to the Ca2+ mobilization studies, experiments assessing inositol-1,4,5-trisphosphate (IP3) accumulation indicated that the presence of micromolar concentrations of either Q94 or Q109 resulted in a significant decrease in thrombin's maximal stimulation (Emax). The antagonistic properties of Q94 and Q109 appear selective as they affected the concentration–response curve of a selective PAR1 activating peptide (AP) while not altering that of a selective PAR2-AP. In addition, these benzimidazole derivatives did not reverse the inhibitory effect of thrombin on isoprenaline- or forskolin-stimulated cAMP production, suggesting they can selectively inhibit the Gq pathway. Importantly, our studies reveal that although the benzimidazole derivatives Q94 and Q109 behave as selective modulators of PAR1 signalling and represent a novel scaffold for the development of new PAR1 inhibitors, their effect on PAR1 signalling is more complex than simple inhibition of Gq activation.
3. IKKβの阻害について
1-Dehydro-[10]-gingerdione from ginger inhibits IKKβ activity for NF-κB activation and suppresses NF-κB-regulated expression of inflammatory genes (pages 128–140)
Hwa Young Lee, Sun Hong Park, Misoon Lee, Hye-Jin Kim, Shi Yong Ryu, Nam Doo Kim, Bang Yeon Hwang, Jin Tae Hong, Sang-Bae Han and Youngsoo Kim
Article first published online: 3 AUG 2012 | DOI: 10.1111/j.1476-5381.
注意事項
Genetic substitutions of the activation loop Ser177 and Ser181 residues with Ala decrease IKKβ activity, whereas those of IKKβ (SS/EE) stimulate kinase activity by mimicking Ser phosphorylation (Mercurio et al., 1997; 1999). Interestingly, IKKβ (C/A) decreases its kinase activity, suggesting that Cys179 plays an important role in the binding affinity of cofactor ATP with IKKβ and in the Ser phosphorylation of IKKβ to stimulate its kinase activity (Byun et al., 2006). To further understand the molecular mechanism of D10G, we proposed molecular docking of D10G to the crystal structure of human IKKβ. The activation loop of IKKβ was somewhat flexible by itself but subtly rearranged to an extended structure upon irreversible binding of D10G with the Cys179 under the most energetically favourable simulation. This conformational change might contribute to the inhibitory mechanism of D10G on IKKβ activity.
BACKGROUND AND PURPOSE
Pungent constituents of ginger (Zingiber officinale) have beneficial effects on inflammatory pain and arthritic swelling. However, the molecular basis for these pharmacological properties is only partially understood. Here, we investigated the molecular target of 1-dehydro-[10]-gingerdione (D10G), one of the pungent constituents of ginger, that mediates its suppression of NF-κB-regulated expression of inflammatory genes linked to toll-like receptor (TLR)-mediated innate immunity.
EXPERIMENTAL APPROACH
RAW 264.7 macrophages or primary macrophages-derived from bone marrows of C57BL/6 or C3H/HeJ mice were stimulated with the TLR4 agonist LPS in the presence of D10G. Catalytic activity of inhibitory κB (IκB) kinase β (IKKβ) was determined by a kinase assay and immunoblot analysis, and the expression of inflammatory genes by RT-PCR analysis and a promoter-dependent reporter assay.
KEY RESULTS
D10G directly inhibited the catalytic activity of cell-free IKKβ. Moreover, D10G irreversibly inhibited cytoplasmic IKKβ-catalysed IκBα phosphorylation in macrophages activated by TLR agonists or TNF-α, and also IKKβ vector-elicited NF-κB transcriptional activity in these cells. These effects of D10G were abolished by substitution of the Cys179 with Ala in the activation loop of IKKβ, indicating a direct interacting site of D10G. This mechanism was shown to mediate D10G-induced disruption of NF-κB activation in LPS-stimulated macrophages and the suppression of NF-κB-regulated gene expression of inducible NOS, COX-2 and IL-6.
CONCLUSION AND IMPLICATIONS
This study demonstrates that IKKβ is a molecular target of D10G involved in the suppression of NF-κB-regulated gene expression in LPS-activated macrophages; this suggests D10G has therapeutic potential in NF-κB-associated inflammation and autoimmune disorders.
彼らのMethods記載
Cell culture
RAW 264.7 macrophages were purchased from ATCC (Manassas, VA). Primary macrophages were prepared from bone marrows of C57BL/6 or C3H/HeJ mice as described previously (Chung et al., 2010). All studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010;McGrath et al., 2010). Animal experiments were carried out following the protocols approved by Animal Experimentation Ethics Committee in CBNU institute. Macrophages were grown in DMEM containing 10% FBS, benzylpenicillin potassium (143 U·mL-1) and streptomycin sulfate (100 µg·mL-1) at 37°C and 5% CO2. RAW 267.4 cells harbuoring the pNF-κB-SEAP-NPT construct were cultured in the same media with an additional supplement of geneticin (500 µg·mL-1).
IKKβ kinase assay
Ser/Thr kinase activity of IKKβ was determined as described previously (Kim et al., 2008). In brief, IKKβ proteins were reacted with substrate GST-IκB (2 µg) and co-factor [γ-32P]-ATP (5 µCi) in a kinase buffer (20 mM HEPES, pH 7.7, 2 mM MgCl2, 50 µM ATP, 10 mM β-glycerophosphate, 10 mM NaF, 300 µg·mL-1 Na3VO4, 2 µM PMSF, 10 µg·mL-1 aprotinin, 1 µg·mL-1 leupeptin, 1 µg·mL-1 pepstatin) at 30°C for 1 h. These reaction mixtures were resolved on SDS-acrylamide gels by electrophoresis. Radioactive bands from the dried gels were then visualized by exposure to X-ray film. Wild-type IKKβ proteins were purchased from Millipore (Billerica, MA, USA). For preparing point-substituted IKKβ proteins, RAW 264.7 cells were transfected with FLAG-tagged expression vector encoding IKKβ (SS/EE) with Glu residues instead of Ser177 and Ser181 or IKKβ (C/A) with Ala residue instead of Cys179 (Kim et al., 2008). Point-substituted IKKβ proteins were precipitated from cell extracts using anti-FLAG affinity gel freezer-safe beads, washed with 20 mM HEPES (pH 7.7) and then subjected to the kinase assay.
4. 鮭カルシトニンでDM治療
Oral salmon calcitonin attenuates hyperglycaemia and preserves pancreatic beta-cell area and function in Zucker diabetic fatty rats (pages 151–163)
M Feigh, KV Andreassen, AV Neutzsky-Wulff, ST Petersen, C Hansen, AC Bay-Jensen, JE Henriksen, H Beck-Nielsen, C Christiansen, K Henriksen and MA Karsdal
Article first published online: 3 AUG 2012 | DOI: 10.1111/j.1476-5381.2012.
BACKGROUND AND PURPOSE
Oral salmon calcitonin (sCT), a dual-action amylin and calcitonin receptor agonist, improved glucose homeostasis in diet-induced obese rats. Here, we have evaluated the anti-diabetic efficacy of oral sCT using parameters of glycaemic control and beta-cell morphology in male Zucker diabetic fatty (ZDF) rats, a model of type 2 diabetes.
EXPERIMENTAL APPROACH
Male ZDF rats were treated with oral sCT (0.5, 1.0 or 2 mg·kg-1) or oral vehicle twice daily from age 8 to 18 weeks. Zucker lean rats served as control group. Fasting and non-fasted blood glucose, glycosylated haemoglobin (HbA1c) and levels of pancreas and incretin hormones were determined. Oral glucose tolerance test and i.p. glucose tolerance test were compared, and beta-cell area and function were evaluated.
KEY RESULTS
Oral sCT treatment dose-dependently attenuated fasting and non-fasted hyperglycaemia during the intervention period. At the end of the study period, oral sCT treatment by dose decreased diabetic hyperglycaemia by ∼9 mM and reduced HbA1c levels by 1.7%. Furthermore, a pronounced reduction in glucose excursions was dose-dependently observed for oral sCT treatment during oral glucose tolerance test. In addition, oral sCT treatment sustained hyperinsulinaemia and attenuated hyperglucagonaemia and hypersecretion of total glucagon-like peptide-1 predominantly in the basal state. Lastly, oral sCT treatment dose-dependently improved pancreatic beta-cell function and beta-cell area at study end.
CONCLUSIONS AND IMPLICATIONS
Oral sCT attenuated diabetic hyperglycaemia in male ZDF rats by improving postprandial glycaemic control, exerting an insulinotropic and glucagonostatic action in the basal state and by preserving pancreatic beta-cell function and beta-cell area.



御礼 誕生日ケーキ・祝賀

敗血症罹患率は,急性心筋梗塞以上に高く,そしてさまざまな内科系病態やがん患者さんの死亡が敗血症であることを理解するための世界DAYが設定されます。
敗血症罹患に気がつくのが遅い,あるいは敗血症と気がついていない,そして敗血症予防の重要性を,これまでも私は指摘してきました。敗血症の診断ができていない,敗血症を予防できていない,抗菌薬使用までの時間が発症から1時間以上に遅い,敗血症罹患後に低タンパクを進行させている,経腸栄養ができていない,リハビリテーションが遅い,嚥下評価などと言っているうちに嚥下障害を作っているなど,非常の遅いスピードの診療が,敗血症診療の質を低下させ,治療を後手に回らせて,死亡率を高めています。今回,World Sepsis Dayは,Stop Sepsis, Save Livesとして,世界の敗血症専門家達の間で合意されたモニュメントとして毎年,9月13日に開催される方針となりました。私自身は,大変な活動ではあるものの,日本にこの9・13 World Sepsis Dayを広めようと思いますし,名古屋でも9・13 World Sepsis Dayを本年より簡易ながらも開催したいと考えています。 Reinhart先生,Opal先生のみならず,Vincent先生,Dellinger先生をふくめて,そうそうたるメンバーが集まっています。

World Sepsis Day
開催 2012年9月13日より毎年9月13日にモニュメントを行う
目的 SSSL: Stop Sepsis, Save Lives
イベント LOS: Lights of Survival 4時間キャンドルを名古屋駅前に陳列して炎をともす,この光が消えぬうちに治療を完了する・・抗菌薬を投与する・・君に何ができるのか!
公共性:good stories supported by strong images
1. The Center of Sepsis Control and Care (CSCC) at the Jena University Hospital in partnership with the Global Sepsis Alliance and the German Sepsis Society initiated the Medical Education for Sepsis Source Control and Antibiotics MEDUSA trial.

Contact: Konrad Reinhart Konrad.Reinhart@med.uni-jena.de
Frank Bloos frank.bloos@med.uni-jena.de
Worldwide, approximately 40% of all patients do not receive treatment according to current recommendations and guidelines of medical societies. Furthermore, a considerable gap exists between ICU directors' perceptions and practiced adherence with evidence-based guidelines. These guidelines of the Surviving Sepsis Campaign (SSC) recommend application of intravenous broad-spectrum antibiotics within 1 hour after diagnosis of sepsis. Although some of the recommendations are controversial, several single-center studies including a study by CSCC investigators supported the hypothesis that quality-improvement efforts based on the SSC guidelines are associated with better outcome.
The primary study objective is to investigate whether a multifaceted educational program with a focus on the early adequate antimicrobial therapy in patients with severe sepsis and septic shock reduces 28 days mortality.
MEDUSA is multicenter, cluster randomized trial limited to hospitals located in Germany. On the patient level, it is an observational study. Inclusion criteria: Involvement in the care of patients with severe sepsis, willingness to participate in quality improvement. Patients: New onset of suspected severe sepsis or septic shock in one of the following settings: prehospital, emergency department, operation theatre, normal ward, intensive care unit / intermediate care unit.
So far the 40 participating hospitals from all over Germany enrolled over 700 patients.
2. Committee to Revise Molecular Definition

Chairman: Steven Opal Opal@brown.edu
Proposed Members: K. Tracey, S Warren, E. Giamarello-Bourbolis and all ISF members.
The molecular definition needs to account for the following findings:
1) The same pattern recognition receptors and signaling pathways are activated by microbial pathogens (TLRs, NLDs, etc) and by host derived Danger (or Damage)-associated molecular pattern molecules (also known as alarmins). This accounts for the difficulty in distinguishing the systemic response from severe infection from severe trauma, burns, ischemia, pancreatitis, etc)
2) The systemic host response to sepsis is highly variable and is attributable to a complex network of cellular and humoral inflammatory mediators, neuroedocrine factors, vasoactive substances and coagulation factors that jointly participate in microvascular injury, increased vascular permeability, organ dysfunction and septic shock.
3) Multiple predisposing conditions, age, gender, genetic factors, medications, nutritional, site of infection and type of infection, and management choices all contribute to the pattern of organ dysfunction and risk of death following severe infection.
And 4) the immune response is dynamic and exists in a spectrum from hyper-inflammatory to highly immunosuppressed.
Correct treatment requires a real time assessment and rx depending on patient needs.
The International Sepsis Forum has decided to devote one of their ISF Colloquia to this topic and will be inviting all stakeholders to attend. Input from all members of the GSA is sought in preparation.
Paul E Marik MD
Crit Care. 2011; 15(3): 158.
PMCID: PMC3218964
Glucocorticoids in sepsis: dissecting facts from fiction

An intact hypothalamic-pituitary-adrenal (HPA) axis with effective intracellular glucocorticoid anti-inflammatory activity is essential for host survival following exposure to an infectious agent. Glucocorticoids play a major role in regulating the activity of nuclear factorkappa- B, which has a crucial and generalized role in inducing cytokine gene transcription after exposure to an invading pathogen. Severe sepsis is, however, associated with complex alterations of the HPA axis, which may result in decreased production of cortisol as well as glucocorticoid tissue resistance.
***************************
Inadequate intracellular glucocorticoid activity, referred to as critical illness-related corticosteroid insufficiency, typically results in an exaggerated proinflammatory response [1]. Patients with severe sepsis or septic shock are therefore frequently treated with exogenous glucocorticoids. While there are large geographic variations in the prescription of glucocorticoids for sepsis, up to 50% of intensive care unit patients receive such therapy [2]. Despite over 30 years of investigation and over 20 meta-analyses, the use of glucocorticoids in patients with sepsis remains extremely controversial and recommendations are conflicting.
The most important recent studies are that of Annane and colleagues [3] and the Corticosteroid Therapy of Septic Shock (CORTICUS) study [4]. Both of these studies have important limitations: 24% patients received etomidate in the study by Annane and colleagues, whereas 19% received etomidate in the CORTICUS study. The benefit of steroids in the study by Annane and colleagues may have been restricted largely to those patients who received etomidate [5]. Furthermore, only patients with 'refractory septic shock' were enrolled in the Annane study whereas, as a result of an overwhelming selection bias, only approximately 5% of eligible patients were enrolled in the CORTICUS study [6]. A more recent study found no benefit from a 7-day course of 40 mg of prednisolone in patients hospitalized with community-acquired pneumonia [7].
In the study by Annane and colleagues [3], patients received 50 mg of hydrocortisone intravenously every 6 hours for 7 days, whereas in the CORTICUS study [4], patients received this dose for 5 days, followed by a tapering off over a further 5 days. Recently, two longitudinal studies in patients with severe community-acquired pneumonia found high levels of circulating inflammatory cytokines 3 weeks after clinical resolution of sepsis [8,9]. These data suggest that patients with severe sepsis may have prolonged immune dysregulation (even after clinical recovery) and that a longer course of corticosteroids may be required. The use of a continuous infusion of hydrocortisone has been reported to result in better glycemic control with less variability of blood glucose concentration [10]. This may be clinically relevant as it has been demonstrated that an oscillating blood glucose level is associated with greater oxidative injury than sustained hyperglycemia [11]. Indeed, a number of reports indicate that glucose variability may be an independent predictor of outcome in critically ill patients [12]. A continuous infusion of glucocorticoid may, however, result in greater suppression of the HPA axis. Furthermore, different glucocorticoids differentially affect gene transcription and have differing pharmacodynamic effects. Consequently, the preferred glucocorticoid and the optimal dosing strategy in patients with septic shock remain to be determined.
Evidence-based medicine is defined as the use of the best current scientific evidence in making decisions about the care of individual patients. Owing to the dearth of high-level evidence, it is not possible to make strong evidence-based recommendations on the use of glucocorticoids in patients with sepsis. Therefore, at this juncture, it is useful to summarize what we know, what we think we know, and what we do not know in order to lay the foundation for future scientific exploration; this information is summarized in Table 1.
In summary, the risk/benefit ratio of glucocorticoids should be determined in each patient. A course (7 to 10 days) of low-dose hydrocortisone (200 mg/day) should be considered in vasopressor-dependent patients (dosage of norepinephrine or equivalent of greater than 0.1 μg/kg per minute) within 12 hours of the onset of shock [1]. Steroids should be stopped in patients whose vasopressor dependency has not improved with 2 days of glucocorticoids. While the outcome benefit of low-dose glucocorticoids remains to be determined, such a strategy decreases vasopressor dependency and appears to be safe (no excess mortality, superinfections, or acute myopathy). Infection surveillance is critical in patients treated with corticosteroids, and to prevent the rebound phenomenon, the drug should be weaned slowly. At this time, glucocorticoids appear to have a limited role in patients who have sepsis or severe sepsis and who are at a low risk of dying.
Table 1
Current knowledge concerning glucocorticoids in sepsis
ステロイド療法における今後の展開が,よくまとめられています。
What we know
• Sepsis causes complex alterations of the hypothalamic-pituitary-adrenal axis and glucocorticoid signaling [1].
• Etomidate causes suppression of cortisol synthesis for up to 24 hours [13].
• High random cortisol levels are a marker of disease severity and a poor prognostic marker [14].
• Short-course, high-dose glucocorticoids are not beneficial in the treatment of severe sepsis/septic shock [15-17].
• Treatment of septic shock with moderate-dose glucocorticoids for 7 days significantly reduces vasopressor dependency (adrenocorticotropin responders and non-responders) and intensive care unit length of stay [15-17].
• Glucocorticoids do not increase the risk of superinfections [15-17].
What we think we know
• Glucocorticoids may reduce mortality in subgroups of patients with septic shock [15-17].
• Glucocorticoids appear to be of no benefit in community-acquired pneumonia patients who are at a low risk of dying [7].
• The addition of fludrocortisone does not appear to have additional benefits when treating patients with hydrocortisone [18].
• Treatment with glucocorticoids may reduce the risk of post-traumatic stress disorder [19].
What we do not know
• Which patients with severe sepsis/septic shock should be treated with glucocorticoids?
• Should treatment with glucocorticoids be based on the results of a cosyntropin stimulation test?
• What is the treatment window? Twenty-four hours?
• How does one accurately diagnose adrenal insufficiency and inadequate cellular glucocorticoid activity?
• What is the optimal dosing schedule of glucocorticoids?
• Which glucocorticoid - methylprednisolone or hydrocortisone - should be used?
• Do glucocorticoids cause long-term myopathy?
• Do we need to treat a patient with glucocorticoids if he or she has received etomidate in the previous 24 hours?
References
1. Marik PE. Critical illness related corticoseroid insufficiency. Chest. 2009;135:181–193. doi: 10.1378/chest.08-1149. [PubMed] [Cross Ref]
2. Beale R, Janes JM, Brunkhorst FM, Dobb G, Levy MM, Martin GS, Ramsey G, Silva E, Sprung C. Global utilization of low-dose corticosteroids in severe sepsis and septic shock: a report from the PROGRESS registry. Crit Care. 2010;14:R102. doi: 10.1186/cc8334. [PMC free article] [PubMed] [Cross Ref]
3. Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaut P, Bellissant E. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [PubMed] [Cross Ref]
4. Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [PubMed] [Cross Ref]
5. Murray H, Marik PE. Etomidate for endotracheal intubation in sepsis: acknowledging the good while accepting the bad. Chest. 2005;127:707–709. doi: 10.1378/chest.127.3.707. [PubMed] [Cross Ref]
6. Marik PE, Pastores SM, Kavanaugh BP. Selection bias negates conclusions from the CORTICUS study? N Engl J Med. 2008;358:2069–2070. [PubMed]
7. Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med. 2010;181:975–982. doi: 10.1164/rccm.200905-0808OC. [PubMed] [Cross Ref]
8. Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [PubMed] [Cross Ref]
9. Lekkou A, Karakantza M, Mouzaki A, Kalfarentzos F, Gogos CA. Cytokine production and monocyte HLA-DR expression as predictors of outcome for patients with community-acquired severe infections. Clin Diagn Lab Immunol. 2004;11:161–167. [PMC free article] [PubMed]
10. Weber-Carstens S, Deja M, Bercker S, Dimroth A, Ahlers O, Kaisers U, Keh D. Impact of bolus application of low-dose hydrocortisone on glycemic control in septic shock patients. Intensive Care Med. 2007;33:730–733. doi: 10.1007/s00134-007-0540-3. [PubMed] [Cross Ref]
11. Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–1354. doi: 10.2337/db08-0063. [PubMed] [Cross Ref]
12. Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105:244–252. doi: 10.1097/00000542-200608000-00006. [PubMed] [Cross Ref]
13. Vinclair M, Broux C, Faure C, Brun J, Gentry C, Jacquot C, Chabre O, Payen JF. Duration of adrenal inhibition following a single dose of etomidate in critically ill patients. Intensive Care Med. 2008;34:714–719. doi: 10.1007/s00134-007-0970-y. [PubMed] [Cross Ref]
14. Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283:1038–1045. doi: 10.1001/jama.283.8.1038. [PubMed] [Cross Ref]
15. Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R, Keh D, Kupfer Y, Oppert M, Meduri GU. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [PMC free article] [PubMed] [Cross Ref]
16. Moran JL, Graham PL, Rockliff S, Bersten AD. Updating the evidence for the role of corticosteroids in severe sepsis and shock: a Bayesian metaanalytic perspective. Crit Care. 2010;14:R134. doi: 10.1186/cc9182. [PMC free article] [PubMed] [Cross Ref]
17. Sligl WI, Milner DA, Sundarr S, Mphatswe W, Majumdar SR. Safety and efficacy of corticosteroids for the treatment of septic shock: a systematic review and meta-analysis. Clin Infect Dis. 2009;49:93–101. doi: 10.1086/599343. [PubMed] [Cross Ref]
18. COIITSS Study Investigators. Annane D, Cariou A, Maxime V, Azoulay E, D'honneur G, Timsit JF, Cohen Y, Wolf M, Fartoukh M, Adrie C, Santré C, Bollaert PE, 17. Mathonet A, Amathieu R, Tabah A, Clec'h C, Mayaux J, Lejeune J, Chevret S. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA. 2010;303:341–348. [PubMed]
19. Schelling G, Briegel J, Roozendaal B, Stoll C, Rothenhäusler HB, Kapfhammer HP. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry. 2001;50:978–985. doi: 10.1016/S0006-3223(01)01270-7.
1.Barrier-protective integrin αvβ3-IQGAP1-Rac1/CDC42-GTP
M. Bhattacharya, G. Su, X. Su, J. A. Oses-Prieto, J. T. Li, X. Huang, H. Hernandez, A. Atakilit, A. L. Burlingame, M. A. Matthay, and D. Sheppard
IQGAP1 is necessary for pulmonary vascular barrier protection in murine acute lung injury and pneumonia
Am J Physiol Lung Cell Mol Physiol July 1, 2012 303:L12-L19
We recently reported that integrin αvβ3 is necessary for vascular barrier protection in mouse models of acute lung injury and peritonitis. Here, we used mass spectrometric sequencing of integrin complexes to isolate the novel β3-integrin binding partner IQGAP1. Like integrin β3, IQGAP1 localized to the endothelial cell-cell junction after sphingosine-1-phosphate (S1P) treatment, and IQGAP1 knockdown prevented cortical actin formation and barrier enhancement in response to S1P. Furthermore, knockdown of IQGAP1 prevented localization of integrin αvβ3 to the cell-cell junction. Similar to β3-null animals, IQGAP1-null mice had increased pulmonary vascular leak compared with wild-type controls 3 days after intratracheal LPS. In an Escherichia coli pneumonia model, IQGAP1 knockout mice had increased lung weights, lung water, and lung extravascular plasma equivalents of 125I-labeled albumin compared with wild-type controls. Taken together, these experiments indicate that IQGAP1 is necessary for S1P-mediated vascular barrier protection during acute lung injury and is required for junctional localization of the barrier-protective integrin αvβ3
2. ビタミンCで肺血管透過性改善?
Bernard J. Fisher, Donatas Kraskauskas, Erika J. Martin, Daniela Farkas, Jacob A. Wegelin, Donald Brophy, Kevin R. Ward, Norbert F. Voelkel, Alpha A. Fowler III, and Ramesh Natarajan
Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid
Am J Physiol Lung Cell Mol Physiol July 1, 2012 303:L20-L32
Bacterial infections of the lungs and abdomen are among the most common causes of sepsis. Abdominal peritonitis often results in acute lung injury (ALI). Recent reports demonstrate a potential benefit of parenteral vitamin C [ascorbic acid (AscA)] in the pathogenesis of sepsis. Therefore we examined the mechanisms of vitamin C supplementation in the setting of abdominal peritonitis-mediated ALI. We hypothesized that vitamin C supplementation would protect lungs by restoring alveolar epithelial barrier integrity and preventing sepsis-associated coagulopathy. Male C57BL/6 mice were intraperitoneally injected with a fecal stem solution to induce abdominal peritonitis (FIP) 30 min prior to receiving either AscA (200 mg/kg) or dehydroascorbic acid (200 mg/kg). Variables examined included survival, extent of ALI, pulmonary inflammatory markers (myeloperoxidase, chemokines), bronchoalveolar epithelial permeability, alveolar fluid clearance, epithelial ion channel, and pump expression (aquaporin 5, cystic fibrosis transmembrane conductance regulator, epithelial sodium channel, and Na+-K+-ATPase), tight junction protein expression (claudins, occludins, zona occludens), cytoskeletal rearrangements (F-actin polymerization), and coagulation parameters (thromboelastography, pro- and anticoagulants, fibrinolysis mediators) of septic blood. FIP-mediated ALI was characterized by compromised lung epithelial permeability, reduced alveolar fluid clearance, pulmonary inflammation and neutrophil sequestration, coagulation abnormalities, and increased mortality. Parenteral vitamin C infusion protected mice from the deleterious consequences of sepsis by multiple mechanisms, including attenuation of the proinflammatory response, enhancement of epithelial barrier function, increasing alveolar fluid clearance, and prevention of sepsis-associated coagulation abnormalities. Parenteral vitamin C may potentially have a role in the management of sepsis and ALI associated with sepsis.
3. Epithelial-Mesenchymal TransitionにおけるTwist & Smail
Epithelial-Mesenchymal Transition (EMT;上皮間葉移行) は1980年代初めにElizabeth Hayらが提唱した上皮細胞が間葉系様細胞に形態変化する現象であり,初期胚発生における原腸陥入,神経提細胞の運動や器官形成過程特に,心臓や腎臓また口蓋形成での重要性がこれまでに明らかとなっている1。一方,EMTの獲得が運動性の亢進や細胞外基質の蓄積をもたらすことから,癌細胞の浸潤や線維症との関連も示唆されている。TwistはbHLH型転写因子で,myogenin プロモーター上でE12とヘテロ2量体を形成し,遺伝子発現を抑制し,さらにSeathre-Chotzen syndromeの原因遺伝子として知られている。Twistの効果は,細胞特異性や他の補助的分子の存在が必要となる可能性が示唆されている。
Koji Sakamoto, Naozumi Hashimoto, Yasuhiro Kondoh, Kazuyoshi Imaizumi, Daisuke Aoyama, Takashi Kohnoh, Masaaki Kusunose, Motohiro Kimura, Tsutomu Kawabe, Hiroyuki Taniguchi, and Yoshinori Hasegawa
Differential modulation of surfactant protein D under acute and persistent hypoxia in acute lung injury
Am J Physiol Lung Cell Mol Physiol July 1, 2012 303:L43-L53
Hypoxia contributes to the development of fibrosis with epithelial-mesenchymal transition (EMT) via stimulation of hypoxia-inducible factor 1α (HIF-1α) and de novo twist expression. Although hypoxemia is associated with increasing levels of surfactant protein D (SP-D) in acute lung injury (ALI), the longitudinal effects of hypoxia on SP-D expression in lung tissue injury/fibrosis have not been fully evaluated. Here, the involvement of hypoxia and SP-D modulation was evaluated in a model of bleomycin-induced lung injury. We also investigated the molecular mechanisms by which hypoxia might modulate SP-D expression in alveolar cells, by using a doxycycline (Dox)-dependent HIF-1α expression system. Tissue hypoxia and altered SP-D levels were present in bleomycin-induced fibrotic lesions. Acute hypoxia induced SP-D expression, supported by the finding that Dox-induced expression of HIF-1α increased SP-D expression. In contrast, persistent hypoxia repressed SP-D expression coupled with an EMT phenotype and twist expression. Long-term expression of HIF-1α caused SP-D repression with twist expression. Ectopic twist expression repressed SP-D expression. The longitudinal observation of hypoxia and SP-D levels in ALI in vivo was supported by the finding that HIF-1α expression stabilized by acute hypoxia induced increasing SP-D expression in alveolar cells, whereas persistent hypoxia induced de novo twist expression in these cells, causing repression of SP-D and acquisition of an EMT phenotype. Thus this is the first study to demonstrate the molecular mechanisms, in which SP-D expression under acute and persistent hypoxia in acute lung injury might be differentially modulated by stabilized HIF-1α expression and de novo twist expression.
4. Ovalbumin 誘導喘息モデル
Kimitake Tsuchiya, Sana Siddiqui, Paul-André Risse, Nobuaki Hirota, and James G. Martin
The presence of LPS in OVA inhalations affects airway inflammation and AHR but not remodeling in a rodent model of asthma
Am J Physiol Lung Cell Mol Physiol July 1, 2012 303:L54-L63
Ovalbumin (OVA) is the most frequently used allergen in animal models of asthma. Lipopolysaccharide (LPS) contaminating commercial OVA may modulate the evoked airway inflammatory response to OVA. However, the effect of LPS in OVA on airway remodeling, especially airway smooth muscle (ASM) has not been evaluated. We hypothesized that LPS in commercial OVA may enhance allergen-induced airway inflammation and remodeling. Brown Norway rats were sensitized with OVA on day 0. PBS, OVA, or endotoxin-free OVA (Ef-OVA) was instilled intratracheally on days 14, 19, 24. Bronchoalveolar lavage (BAL) fluid, lung, and intrathoracic lymph node tissues were collected 48 h after the last challenge. Immunohistochemistry for α-smooth muscle actin, Periodic-Acid-Schiff staining, and real-time qPCR were performed. Airway hyperresponsiveness (AHR) was also measured. BAL fluid macrophages, eosinophils, neutrophils, and lymphocytes were increased in OVA-challenged animals, and macrophages and neutrophils were significantly lower in Ef-OVA-challenged animals. The ASM area in larger airways was significantly increased in both OVA and Ef-OVA compared with PBS-challenged animals. The mRNA expression of IFN-γ and IL-13 in lung tissues and IL-4 in lymph nodes was significantly increased by both OVA and Ef-OVA compared with PBS and were not significantly different between OVA and Ef-OVA. Monocyte chemoattractant protein (MCP)-1 in BAL fluid and AHR were significantly increased in OVA but not in Ef-OVA. LPS contamination in OVA contributes to the influx of macrophages and MCP-1 increase in the airways and to AHR after OVA challenges but does not affect OVA-induced Th1 and Th2 cytokine expression, goblet cell hyperplasia, and ASM remodeling.
5. 肺高血圧におけるglucose-6-phosphate dehydrogenaseとPKG
Sukrutha Chettimada, Dhwajbahadur K. Rawat, Nupur Dey, Robert Kobelja, Zachary Simms, Michael S. Wolin, Thomas M. Lincoln, and Sachin A. Gupte
Glc-6-PD and PKG contribute to hypoxia-induced decrease in smooth muscle cell contractile phenotype proteins in pulmonary artery
Am J Physiol Lung Cell Mol Physiol July 1, 2012 303:L64-L74
Persistent hypoxic pulmonary vasoconstriction (HPV) plays a significant role in the pathogenesis of pulmonary hypertension, which is an emerging clinical problem around the world. We recently showed that hypoxia-induced activation of glucose-6-phosphate dehydrogenase (Glc-6-PD) in pulmonary artery smooth muscle links metabolic changes within smooth muscle cells to HPV and that inhibition of Glc-6PD reduces acute HPV. Here, we demonstrate that exposing pulmonary arterial rings to hypoxia (20–30 Torr) for 12 h in vitro significantly (P < 0.05) reduces (by 30–50%) SM22α and smooth muscle myosin heavy chain expression and evokes HPV. Glc-6-PD activity was also elevated in hypoxic pulmonary arteries. Inhibition of Glc-6-PD activity prevented the hypoxia-induced reduction in SM22α expression and inhibited HPV by 80–90% (P < 0.05). Furthermore, Glc-6-PD and protein kinase G (PKG) formed a complex in pulmonary artery, and Glc-6-PD inhibition increased PKG-mediated phosphorylation of VASP (p-VASP). In turn, increasing PKG activity upregulated SM22α expression and attenuated HPV evoked by Glc-6-PD inhibition. Increasing passive tension (from 0.8 to 3.0 g) in hypoxic arteries for 12 h reduced Glc-6-PD, increased p-VASP and SM22α levels, and inhibited HPV. The present findings indicate that increases in Glc-6-PD activity influence PKG activity and smooth muscle cell phenotype proteins, all of which affect pulmonary artery contractility and remodeling.
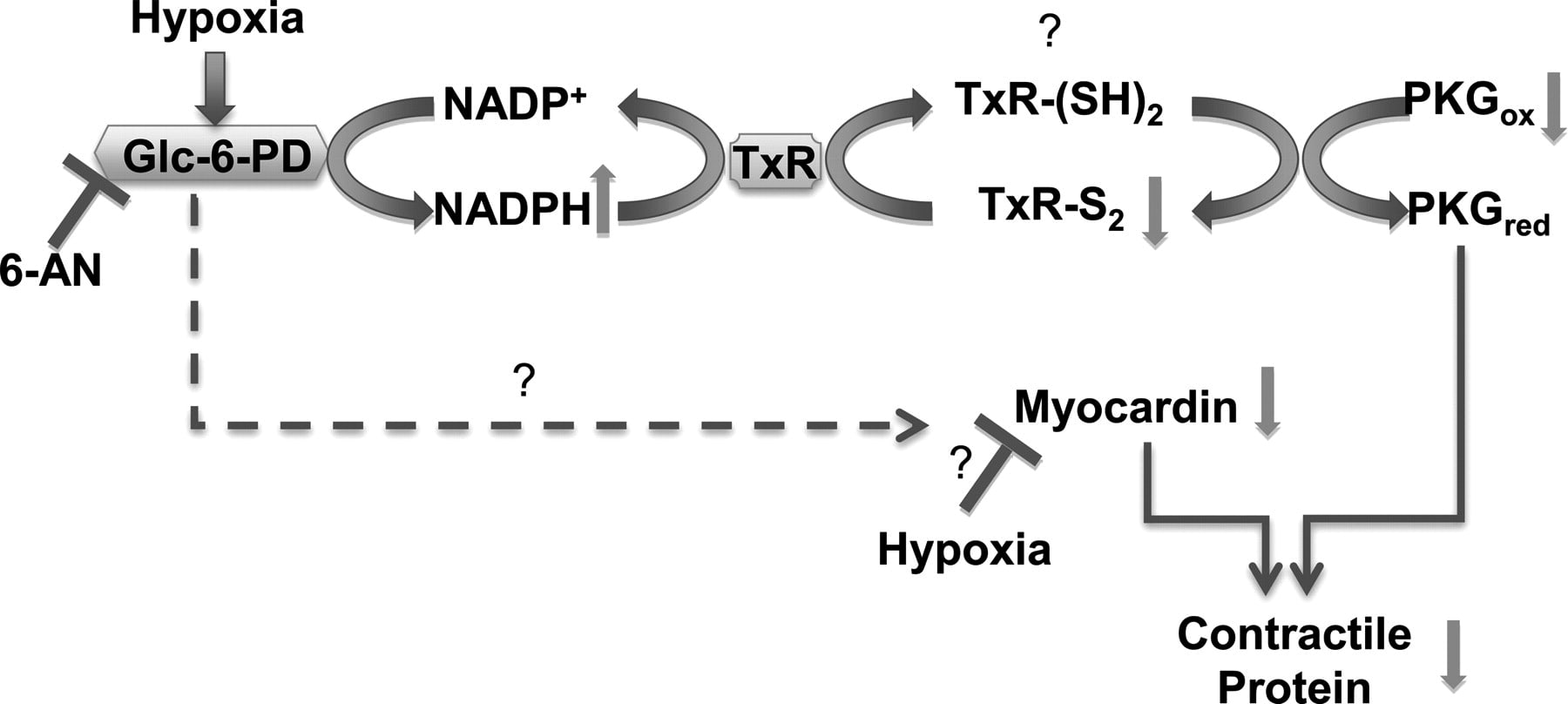
Glc-6-PD and thioredoxin reductase-1 (TxR-1) form a complex with PKG and regulate hypoxia-evoked changes in the expression of contractile proteins in pulmonary artery. Schematic illustrating a potential pathway through which hypoxia affects activity of Glc-6-PD and PKG and expression of contractile proteins. Inhibition of Glc-6-PD-derived NADPH redox reduces TxR-1 activity, which oxidizes thiols on PKG and activates it. Increase in PKG activity reexpresses contractile proteins, whereas myocardin expression is increased in a PKG-independent manner.
Based on the results of several small trials, the safety of colloid solutions containing high-molecular-weight hydroxyethyl starch (HES) has been questioned in patients with severe sepsis. In the July 12 issue of The New England Journal of Medicine, Perner and investigators from the 6S Trial Group and the Scandinavian Critical Care Trials Group reported the results from a multicenter, blinded, stratified, parallel-group randomized trial conducted in 26 Scandinavian intensive care units from 2009 to 2011. The effects of HES (130/0.42) were compared to those of Ringer’s acetate on the composite outcomes of death or end-stage renal failure in patients with severe sepsis.
Computerized allocation was used to blindly randomize 804 patients. Treatment assignments were concealed from investigators, clinicians and patients. Adults older than 18 and fulfilling criteria for severe sepsis were enrolled. The primary outcome was death or dependence on dialysis 90 days after randomization. The study was appropriately powered to detect an absolute difference in the primary outcome, with multivariate analyses used to adjust for differences in baseline variables.
The study followed 398 patients in the HES group and 400 in the Ringer’s group (N=798 total) for at least 90 days. Baseline characteristics were similar in the two groups, with each having an incidence of acute kidney injury over 35%. The median cumulative volume of fluid received was not statistically significantly different between the two groups. More patients in the HES group received blood products and required renal replacement therapy. The primary outcome of death was higher in the HES group (relative risk [RR], 1.17; 95% confidence interval [CI], 1.01 to 1.36; P=0.03); 53% of patients in the HES group died compared to 43% in the Ringer’s group. The authors hypothesized that one explanation for the findings may be that HES is deposited in tissues, and may act as a toxic foreign body.
This well-conducted study conformed to a modified intention-to-treat protocol. Although a high number of patients with acute kidney injury were included, this comorbidity was evenly balanced with the randomized design. Patients received strikingly similar amounts of fluids, though the trial did not employ advanced hemodynamic monitoring or protocols for titrating fluids.
HES was associated with an increase in absolute risk of death (number needed to harm=13). Based on the results of this trial and previous work in this area, the use of HES should be critically examined as an option for colloid resuscitation in patients with severe sepsis. Patients with severe sepsis who received HES were at increased risk of death at 90 days, more likely to receive renal-replacement therapy, and were alive for fewer days once discharged from the hospital.
※ 敗血症は全身性炎症の代表的病態です。このような検証が行われるまでもなく,重症敗血症や敗血症性ショックにHESを使うことはないと思いますがが,これは敗血症にのみ有害なわけではありません。理論的には炎症性手術においてHESを用いれば臓器不全が進行する可能性があり,既に一臓器以上の臓器不全を持っている場合には,90日死亡率にも影響が出る可能性があるということです。特に,術後に腎機能を低下させる可能性については,炎症性サイトカインの増加する手術において,極めてリスクが高いと評価されます。
Hydroxyethyl Starch 130/0.42 versus Ringer's Acetate in Severe Sepsis
N Engl J Med 2012; 367:124-134
Anders Perner, M.D., Ph.D., Nicolai Haase, M.D., Anne B. Guttormsen, M.D., Ph.D., Jyrki Tenhunen, M.D., Ph.D., Gudmundur Klemenzson, M.D., Anders Åneman, M.D., Ph.D., Kristian R. Madsen, M.D., Morten H. Møller, M.D., Ph.D., Jeanie M. Elkjær, M.D., Lone M. Poulsen, M.D., Asger Bendtsen, M.D., M.P.H., Robert Winding, M.D., Morten Steensen, M.D., Pawel Berezowicz, M.D., Ph.D., Peter Søe-Jensen, M.D., Morten Bestle, M.D., Ph.D., Kristian Strand, M.D., Ph.D., Jørgen Wiis, M.D., Jonathan O. White, M.D., Klaus J. Thornberg, M.D., Lars Quist, M.D., Jonas Nielsen, M.D., Ph.D., Lasse H. Andersen, M.D., Lars B. Holst, M.D., Katrin Thormar, M.D., Anne-Lene Kjældgaard, M.D., Maria L. Fabritius, M.D., Frederik Mondrup, M.D., Frank C. Pott, M.D., D.M.Sci., Thea P. Møller, M.D., Per Winkel, M.D., D.M.Sci., and Jørn Wetterslev, M.D., Ph.D. for the 6S Trial Group and the Scandinavian Critical Care Trials Group

BACKGROUND
Hydroxyethyl starch (HES) is widely used for fluid resuscitation in intensive care units (ICUs), but its safety and efficacy have not been established in patients with severe sepsis.
METHODS
In this multicenter, parallel-group, blinded trial, we randomly assigned patients with severe sepsis to fluid resuscitation in the ICU with either 6% HES 130/0.42 (Tetraspan) or Ringer's acetate at a dose of up to 33 ml per kilogram of ideal body weight per day. The primary outcome measure was either death or end-stage kidney failure (dependence on dialysis) at 90 days after randomization.
RESULTS
Of the 804 patients who underwent randomization, 798 were included in the modified intention-to-treat population. The two intervention groups had similar baseline characteristics. At 90 days after randomization, 201 of 398 patients (51%) assigned to HES 130/0.42 had died, as compared with 172 of 400 patients (43%) assigned to Ringer's acetate (relative risk, 1.17; 95% confidence interval [CI], 1.01 to 1.36; P=0.03); 1 patient in each group had end-stage kidney failure. In the 90-day period, 87 patients (22%) assigned to HES 130/0.42 were treated with renal-replacement therapy versus 65 patients (16%) assigned to Ringer's acetate (relative risk, 1.35; 95% CI, 1.01 to 1.80; P=0.04), and 38 patients (10%) and 25 patients (6%), respectively, had severe bleeding (relative risk, 1.52; 95% CI, 0.94 to 2.48; P=0.09). The results were supported by multivariate analyses, with adjustment for known risk factors for death or acute kidney injury at baseline.
CONCLUSIONS
Patients with severe sepsis assigned to fluid resuscitation with HES 130/0.42 had an increased risk of death at day 90 and were more likely to require renal-replacement therapy, as compared with those receiving Ringer's acetate. (Funded by the Danish Research Council and others; 6S ClinicalTrials.gov number, NCT00962156.)
Hydroxyethyl starch (HES) versus other fluid therapies: effects on kidney function.
Cochrane Database Syst Rev. 2010 Jan 20;(1):CD007594.
Dart AB, Mutter TC, Ruth CA, Taback SP.
Source
Department of Pediatrics and Child Health, University of Manitoba, FE-009 840 Sherbrook St, Winnipeg, Manitoba, Canada, R3A 1S1.
Abstract
BACKGROUND:
Hydroxyethyl starches (HES) are synthetic colloids commonly used for fluid resuscitation, yet controversy exists about their impact on kidney function.
OBJECTIVES:
To examine the effects of HES on kidney function compared to other fluid resuscitation therapies in different patient populations.
SEARCH STRATEGY:
We searched the Cochrane Renal Group's specialised register, the Cochrane Central Register of Controlled Trials (CENTRAL, in The Cochrane Library), MEDLINE, EMBASE, MetaRegister and reference lists of articles.
SELECTION CRITERIA:
Randomised controlled trials (RCTs) and quasi-RCTs in which HES was compared to an alternate fluid therapy for the prevention or treatment of effective intravascular volume depletion. Primary outcomes were renal replacement therapy (RRT), author-defined kidney failure and acute kidney injury (AKI) as defined by the RIFLE criteria. Secondary outcomes included serum creatinine and creatinine clearance.
DATA COLLECTION AND ANALYSIS:
Screening, selection, data extraction and quality assessments for each retrieved article were carried out by two authors using standardised forms. Authors were contacted when published data were incomplete. Preplanned sensitivity and subgroup analyses were performed after data were analysed with a random effects model.
MAIN RESULTS:
The review included 34 studies (2607 patients). Overall, the RR of author-defined kidney failure was 1.50 (95% CI 1.20 to 1.87; n = 1199) and 1.38 for requiring RRT (95% CI 0.89 to 2.16; n = 1236) in HES treated individuals compared with other fluid therapies. Subgroup analyses suggested increased risk in septic patients compared to non-septic (surgical/trauma) patients. Non-septic patient studies were smaller and had lower event rates, so subgroup differences may have been due to lack of statistical power in these studies. Only limited data was obtained for analysis of kidney outcomes by the RIFLE criteria. Overall, methodological quality of studies was good but subjective outcomes were potentially biased because most studies were unblinded.
AUTHORS' CONCLUSIONS:
Potential for increased risk of AKI should be considered when weighing the risks and benefits of HES for volume resuscitation, particularly in septic patients. Large studies with adequate follow-up are required to evaluate the renal safety of HES products in non-septic patient populations. RIFLE criteria should be applied to evaluate kidney function in future studies of HES and, where data is available, to re-analyse those studies already published. There is inadequate clinical data to address the claim that safety differences exist between different HES products.
循環血液量増加に関してはアルブミンもHESも有効 by びんちゃん
Su F, Wang Z, Cai Y, Rogiers P, Vincent JL.
Shock. 2007 May;27(5):520-6.
Fluid resuscitation in severe sepsis and septic shock: albumin, hydroxyethyl starch, gelatin or ringer's lactate-does it really make a difference?
Abstract
The aim of this study was to investigate whether the type of i.v. fluid administered has an impact on outcome in an animal model of septic shock. The study included 28 anesthetized, invasively monitored, mechanically ventilated female sheep (29.5 +/- 4.0 kg), which received 0.5 g/kg body weight of feces into the abdominal cavity to induce peritonitis. During the surgical operation and 4 h after feces spillage, only Ringer's lactate (RL) was administered in all animals. Thereafter, animals were randomized to receive continuous infusions of RL (n = 7) alone or combined with either 20% albumin (n = 7, volume ratio to RL 1:10) or 6% hydroxyethyl starch (HES) (n = 7, volume ratio to RL 1:1), or gelatin alone (n= 7, no volume limitation). Fluid resuscitation was titrated to maintain pulmonary artery occlusion pressure at baseline levels throughout the experiment. No antibiotics or vasoactive drugs were administered, and animals were monitored until their spontaneous death. Hemodynamic variables were better with HES and albumin than with the other fluids, as reflected by higher stroke volume, cardiac index, and oxygen delivery (all P < 0.05). Hydroxyethyl-starch-treated animals also had lower arterial lactate concentrations (P < 0.01). However, times to develop hypotension and oliguria were similar in all groups. Blood interleukin (IL) 6 concentrations were significantly increased in all groups. The mean survival time was similar in all groups. In this clinically relevant model of prolonged septic shock, albumin and HES solution resulted in higher cardiac output, oxygen delivery, and lower blood lactate levels than gelatin and RL; however, the choice of i.v. fluid did not affect outcome.
救急医療 急性脱水症
名古屋大学大学院医学系研究科
救急・集中治療医学分野
松田直之
基本知識
急性脱水症は,急激な水摂取低下や水消失により,体内水分量の減少した状態です。脱水では,病歴から原因を検索することが大切であり,水分と塩分の摂取状況を把握することが必要です。
急性脱水症の病態は,塩類消失の有無により,大きく① 高張性脱水,② 等張性脱水,③ 低張性脱水の3つに分類しています。一般に,水摂取低下で血清ナトリウム(Na)濃度が上昇しはじめると,細胞内より細胞外へ水が移動して細胞内脱水となります。このような状況では,視床下部の浸透圧受容器を介して口渇感と水摂取を促されますが,人工呼吸管理や意識障害を伴う場合には水補充の不適正により高張性脱水が導かれやすいです。一方,等張性脱水や低張性脱水では,バソプレシンやアルドステロンの分泌亢進により,腎における水再吸収が促進され,尿が濃縮される傾向があります。
このような状況において,水摂取低下の原因としては,① 意識障害,② 悪心,③ 消化器症状などによる経口摂取不能や水分制限が挙げられます。一方,腎性水喪失の要因としては,① 多尿,② ループ利尿薬,③ 浸透圧性利尿薬,④ 塩類喪失性腎症,⑤ 鉱質コルチコイド分泌低下などを評価します。さらに,腎以外の要因による腎外性水喪失の原因としては,① 嘔吐,② 下痢,③ 全身性炎症に伴う血管透過性亢進病態,④ 消化管浮腫,⑤ 低蛋白血症,⑥ 胸水,⑦ 腹水,⑧ 発熱の推移などを評価します。
脱水の臨床症状は,① 皮膚や舌の乾燥,② 眼球陥没,③ 頻呼吸,④ 尿量の低下と尿比重の上昇です。脱水が進行すると,循環血液量が減少し,意識低下やショックを認めます。血液・生化学検査では,血清Na濃度,血清カリウム(K)濃度,血清クロール濃度,血清総蛋白,血清アルブミン濃度,BUN,ヘマトクリット値,さらに血液ガス分析でまず,貧血,代謝性アシドーシスの進行と血中乳酸値の蓄積を時系列で評価して下さい。
輸液例
循環血液量の減少を認める場合は,細胞外液液を点滴静注します。経口摂取が使用可能であれば,経口補液(ORS:oral rehydration solution)の投与を検討してください。
1. 高張性脱水でない場合
1)ヴィーンF注 500 mL 点滴静注
2)ビカーボン注 500 mL 点滴静注
3)生理食塩液 500 mL 点滴静注
開始時の輸液スピードは,バイタルサインに応じて決定します。必ず呼吸数を時系列でチェックし,カルテに記載を残すようにして下さい。尿量 0.5 mL/kg/時以上が得られることを目標とします。
2.高張性脱水の場合(Kフリーの選択)
1)ソリタ-T1号注 500 mL 点滴静注
2)5%ブドウ糖液注 500 mL 点滴静注
まず,上述の液を初期輸液剤とし,① 利尿がつくこと,② 高カリウム血症でないことを確認します。急性脱水症や大量の輸液量を必要とする場合に,急激な血糖値上昇の可能性があるために,血糖値を評価し,ヴィーンF注 やビカーボン注などの併用も考えます。
知っておくとよい注意事項
1.貧血および輸液量に対する注意
パルスオキシメータ波形の呼吸性変動が強い場合は,脱水を示唆する所見です(救急一直線 講義 パルスオキシメータおよびA-lineの圧波形の読み方)。意識・呼吸・循環,バイタルサインに異常が認められる急性脱水症では,特に非侵襲的気道確保と輸液に注意して下さい。この際に,貧血の程度をいち早くアセスメントして下さい。基本的にはまず,リンゲル液や生理食塩水 1 Lを15分程度で投与し,バイタルサインの改善を初期目標とします。この際には,必ずパルス波形をチェックして下さい。また,貧血(Hb<7 g/dL)における急速輸液は,極端なHb低下,酸素運搬低下,心肺停止を導く可能性がありますので,赤血球輸血を念頭において対応して下さい。
2.高齢者・心機能異常・低栄養を伴う場合
高齢者や,心機能異常,弁疾患,および低栄養を伴う場合には,急激な輸液負荷により肺水腫,胸水,および腹水が増強する場合があります。心エコー検査を時系列で施行し,急速輸液に慎重に対応します。
3. 意識障害における対応:脱水と高ナトリウム血症
意識障害を伴う高度の高張性脱水では,細胞外液量の補正とともに,バゾプレシン分泌低下や浸透圧利尿薬の使用状況を評価すると共に,これらを踏まえて,高Na血症の是正が必要となります。このような場合,血清Na濃度の低下速度が0.5~1 mEq/L/時程度となるように補正します。
4.低Na血症を伴う急性脱水症
低Na血症を伴う急性脱水症では,Naの腎性喪失を評価すると共に,尿中のNaとK濃度の評価が必要です。尿中(Na+K)濃度>血清Na濃度の場合には,細胞外液輸液のみでは低Na血症が進行することに注意します。
5.尿浸透圧・尿中Na濃度の評価
腎外性喪失の場合には,腎機能が正常であれば尿浸透圧は450 mOsm/L以上,尿比重は1.015以上に注意します。また,Na排泄率(FENa=尿中Na濃度/血漿Na濃度)/(尿中クレアチニン濃度/血漿クレアチニン濃度)が1%以下であれば腎外性喪失の可能性があります。
救急科専門医や上級医への紹介のタイミング
意識低下,呼吸数>30回/分 あるいは < 9回/分,頻脈,血圧低下を認める場合は,生命に危険を伴う緊急性の高い状態であり,病棟で入院対応せず,救急・集中治療の専門医にコンサルトするとよいです。


















