1. 総説 Neuronal Norepinephrine Transporter (NET)の異常について
C. Schroeder and J. Jordan
Norepinephrine transporter function and human cardiovascular disease
Am J Physiol Heart Circ Physiol December 1, 2012 303:H1273-H1282
pproximately 80–90% of the norepinephrine released in the brain or in peripheral tissues is taken up again through the neuronal norepinephrine transporter (NET). Pharmacological studies with NET inhibitors showed that NET has opposing effects on cardiovascular sympathetic regulation in the brain and in the periphery. Furthermore, NET is involved in the distribution of sympathetic activity between vasculature, heart, and kidney. Genetic NET dysfunction is a rare cause of the postural tachycardia syndrome. The condition is characterized by excessive adrenergic stimulation of the heart, particularly with standing. Conversely, NET inhibition may be beneficial in hypoadrenergic states, such as central autonomic failure or neurally mediated syncope, which results from acute sympathetic withdrawal. Biochemical studies suggested reduced NET function in some patients with essential hypertension. Furthermore, cardiac NET function appears to be reduced in common heart diseases, such as congestive heart failure, ischemic heart disease, and stress-induced cardiomyopathy. Whether NET dysfunction is a consequence or cause of progressive heart disease in human subjects requires further study. However, studies with the nonselective NET inhibitor sibutramine suggest that reduced NET function could have an adverse effect on the cardiovascular system. Given the widespread use of medications inhibiting NET, the issue deserves more attention.
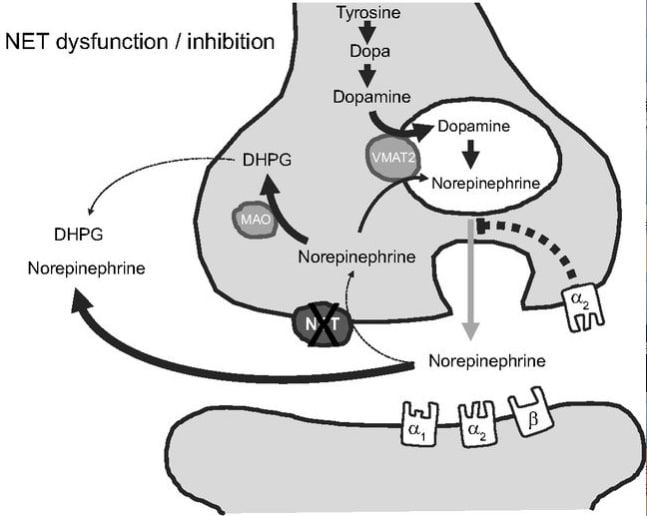
Schematic diagram of norepinephrine biosynthesis, release, reuptake, and degradation. For details see text. DHPG, dihydroxyphenylglycol; NET, norepinephrine reuptake transporter; MAO, monoaminooxidase; VMAT2, vesicular monoamine transporter-2.
2. 総説 ミトコンドリアNOSについて<虚血再潅流研究の知識として重要>
Tamara Zaobornyj and Pedram Ghafourifar
Strategic localization of heart mitochondrial NOS: a review of the evidence
Am J Physiol Heart Circ Physiol December 1, 2012 303:H1283-H129
Heart mitochondria play a central role in cell energy provision and in signaling. Nitric oxide (NO) is a free radical with primary regulatory functions in the heart and involved in a broad array of key processes in cardiac metabolism. Specific NO synthase (NOS) isoforms are confined to distinct locations in cardiomyocytes. The present article reviews the chemical reactions through which NO interacts with biomolecules and exerts some of its crucial roles. Specifically, the article discusses the reactions of NO with mitochondrial targets and the subcellular localization of NOS within the myocardium and analyzes the available data about heart mitochondrial NOS activity and identity. The article also describes the regulation of heart mtNOS by the distinctive mitochondrial environment by showing the effects of Ca2+, O2, L-arginine, mitochondrial transmembrane potential, and the metabolic states on heart mitochondrial NO production. The article depicts the effects of NO on heart function and highlights the relevance of NO production within mitochondria. Finally, the evidence on the functional implications of heart mitochondrial NOS is delineated with emphasis on chronic hypoxia and ischemia-reperfusion studies.
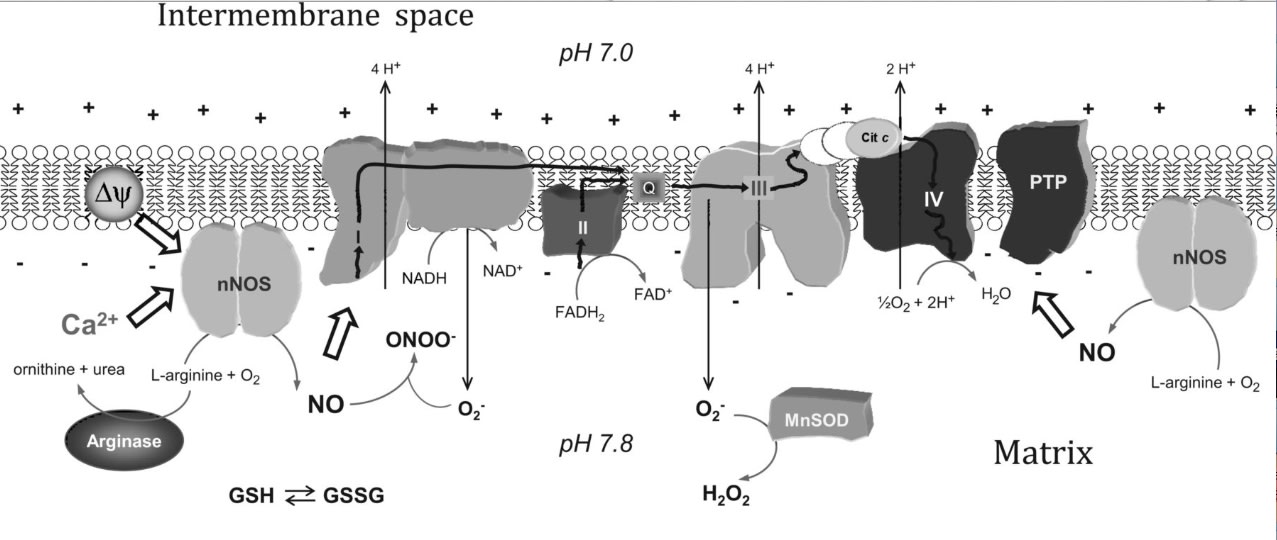
Reciprocal regulation of mtNOS activity and mitochondrial function. Localization of NOS within mitochondria provides a distinct specific regulation of mitochondrial NO production by intramitochondrial O2, Ca2+, pH, mitochondrial transmembrane potential (Δψ), L-arginine, arginases, or redox state (GSH/GSGG balance). NO produced by mtNOS can readily react with mitochondrial targets such as respiratory complexes I, III, or IV or mitochondrial PTP. The superoxide anion (O2-) is formed at the respiratory chain and undergoes a very fast reaction with NO to form peroxynitrite (ONOO-), or it is catabolized by MnSOD to form H2O2. Cit c, cytochrome c.
3. 総説 Circulation miRNAについて
Anke J. Tijsen, Yigal M. Pinto, and Esther E. Creemers
Circulating microRNAs as diagnostic biomarkers for cardiovascular diseases
Am J Physiol Heart Circ Physiol November 1, 2012 303:H1085-H1095
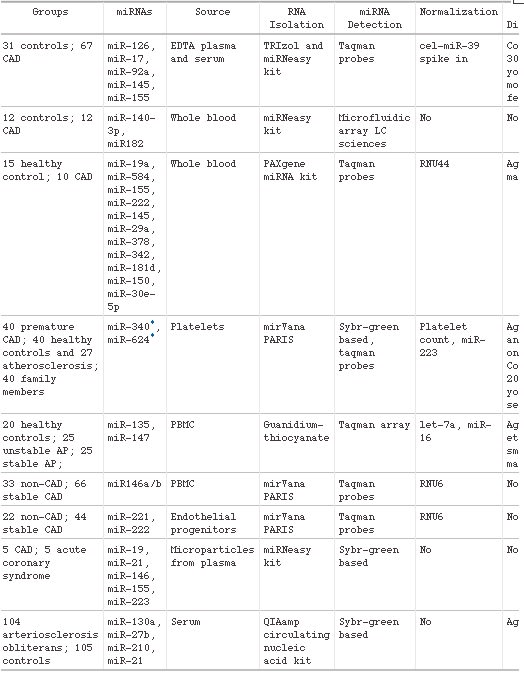
One of the major challenges in cardiovascular disease is the identification of reliable clinical biomarkers that can be routinely measured in plasma. MicroRNAs (miRNAs) were recently discovered to circulate in the bloodstream in a remarkably stable form. Because of their stability and often tissue- and disease-specific expression and the possibility to measure them with high sensitivity and specificity, miRNAs are emerging as new diagnostic biomarkers. In this review we will provide an overview of the potential of circulating miRNAs as biomarkers for a wide range of cardiovascular diseases such as coronary artery disease, myocardial infarction, hypertension, heart failure, viral myocarditis, and type-2 diabetes mellitus. Furthermore, we will discuss the challenges with regard to further validation in large patient cohorts, and we will discuss how the measurement of multiple miRNAs simultaneously might improve the accuracy of the diagnostic test.
Circulating miRNAs as biomarkers for CAD
4. miR-10のFlt-1の発現調節作用
MicroRNAs Control Vascular Endothelial Growth Factor Signaling
Reinier A. Boon
Circ Res 2012 Nov 9;111(11):1388-90

Angiogenesis is a very tightly controlled process, in which endothelial cells need to migrate and proliferate toward ischemic tissue. A long-known factor that provides a gradient for endothelial cells to migrate toward is vascular endothelial growth factor (VEGF).1 Carefully titrated levels of VEGF are crucial for blood vessel development, because even the heterozygous deletion of VEGF is lethal in mice.2 In the past decades, it has become clear that VEGF signaling is very complex, with different VEGF isoforms and VEGF receptors that tightly control endothelial cell behavior. Interestingly, although VEGF receptor 2 (kinase insert domain receptor [KDR]) is one of the main proangiogenic VEGF receptors,3 binding of VEGF to VEGF receptor 1 (fms-related tyrosine kinase 1 [FLT1]) does not result in proangiogenic signaling, which raised the concept that FLT1 acts as a trap or decoy for VEGF. Thus, FLT1 can negatively regulate VEGF signaling, and this is of crucial importance, for instance, to keep the cornea avascular,4 but also aids in controlling the fine balance between proangiogenic and antiangiogenic factors. To make matters more complex, FLT1 mRNA is alternatively spliced, giving rise to a membrane-bound FLT1 and secreted soluble FLT1,5 but they both function as VEGF traps, preventing VEGF from activating KDR. In this issue of Circulation Research, Hassel et al6 report that both isoforms of FLT1 are regulated by the microRNA (miRNA) miR-10.
最新の画像[もっと見る]
-
 お知らせ 世界敗血症デー 2024年9月イベント
4ヶ月前
お知らせ 世界敗血症デー 2024年9月イベント
4ヶ月前
-
 お知らせ 世界敗血症デー 2024年9月イベント
4ヶ月前
お知らせ 世界敗血症デー 2024年9月イベント
4ヶ月前
-
 お知らせ 世界敗血症デー 2024年9月イベント
4ヶ月前
お知らせ 世界敗血症デー 2024年9月イベント
4ヶ月前
-
 お知らせ 世界敗血症デー 2024年9月イベント
4ヶ月前
お知らせ 世界敗血症デー 2024年9月イベント
4ヶ月前
-
 お知らせ 世界敗血症デー 2024年9月イベント
4ヶ月前
お知らせ 世界敗血症デー 2024年9月イベント
4ヶ月前
-
 お知らせ 世界敗血症デー 2024年9月イベント
4ヶ月前
お知らせ 世界敗血症デー 2024年9月イベント
4ヶ月前
-
 セミナーのお知らせ 多発外傷カンファレンス with デューク大学
5ヶ月前
セミナーのお知らせ 多発外傷カンファレンス with デューク大学
5ヶ月前
-
 執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
5ヶ月前
執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
5ヶ月前
-
 執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
5ヶ月前
執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
5ヶ月前
-
 執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
5ヶ月前
執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
5ヶ月前
「ジャーナルクラブ 松田直之指導」カテゴリの最新記事
 ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年12月号 Sonic Hed...
ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年12月号 Sonic Hed... 2012年12月13日 基盤ジャーナルクラブ 心血管基礎研究関連
2012年12月13日 基盤ジャーナルクラブ 心血管基礎研究関連 文献紹介 Hypoglycemia and Risk of Death in Critically Ill Patients N Engl J...
文献紹介 Hypoglycemia and Risk of Death in Critically Ill Patients N Engl J... ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年11月号
ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年11月号 ジャーナルクラブ Circulation Res 2012, September 14 & 28
ジャーナルクラブ Circulation Res 2012, September 14 & 28 ジャーナルクラブ British J Pharmacol 2012. October Volume 167, Issue 3
ジャーナルクラブ British J Pharmacol 2012. October Volume 167, Issue 3 ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年9月号
ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年9月号 ジャーナルクラブ Circulation Research 2012. August
ジャーナルクラブ Circulation Research 2012. August ジャーナルクラブ British Journal of Pharmacology 2012. September
ジャーナルクラブ British Journal of Pharmacology 2012. September ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年7月号
ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年7月号

















