1.Barrier-protective integrin αvβ3-IQGAP1-Rac1/CDC42-GTP
M. Bhattacharya, G. Su, X. Su, J. A. Oses-Prieto, J. T. Li, X. Huang, H. Hernandez, A. Atakilit, A. L. Burlingame, M. A. Matthay, and D. Sheppard
IQGAP1 is necessary for pulmonary vascular barrier protection in murine acute lung injury and pneumonia
Am J Physiol Lung Cell Mol Physiol July 1, 2012 303:L12-L19
We recently reported that integrin αvβ3 is necessary for vascular barrier protection in mouse models of acute lung injury and peritonitis. Here, we used mass spectrometric sequencing of integrin complexes to isolate the novel β3-integrin binding partner IQGAP1. Like integrin β3, IQGAP1 localized to the endothelial cell-cell junction after sphingosine-1-phosphate (S1P) treatment, and IQGAP1 knockdown prevented cortical actin formation and barrier enhancement in response to S1P. Furthermore, knockdown of IQGAP1 prevented localization of integrin αvβ3 to the cell-cell junction. Similar to β3-null animals, IQGAP1-null mice had increased pulmonary vascular leak compared with wild-type controls 3 days after intratracheal LPS. In an Escherichia coli pneumonia model, IQGAP1 knockout mice had increased lung weights, lung water, and lung extravascular plasma equivalents of 125I-labeled albumin compared with wild-type controls. Taken together, these experiments indicate that IQGAP1 is necessary for S1P-mediated vascular barrier protection during acute lung injury and is required for junctional localization of the barrier-protective integrin αvβ3
2. ビタミンCで肺血管透過性改善?
Bernard J. Fisher, Donatas Kraskauskas, Erika J. Martin, Daniela Farkas, Jacob A. Wegelin, Donald Brophy, Kevin R. Ward, Norbert F. Voelkel, Alpha A. Fowler III, and Ramesh Natarajan
Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid
Am J Physiol Lung Cell Mol Physiol July 1, 2012 303:L20-L32
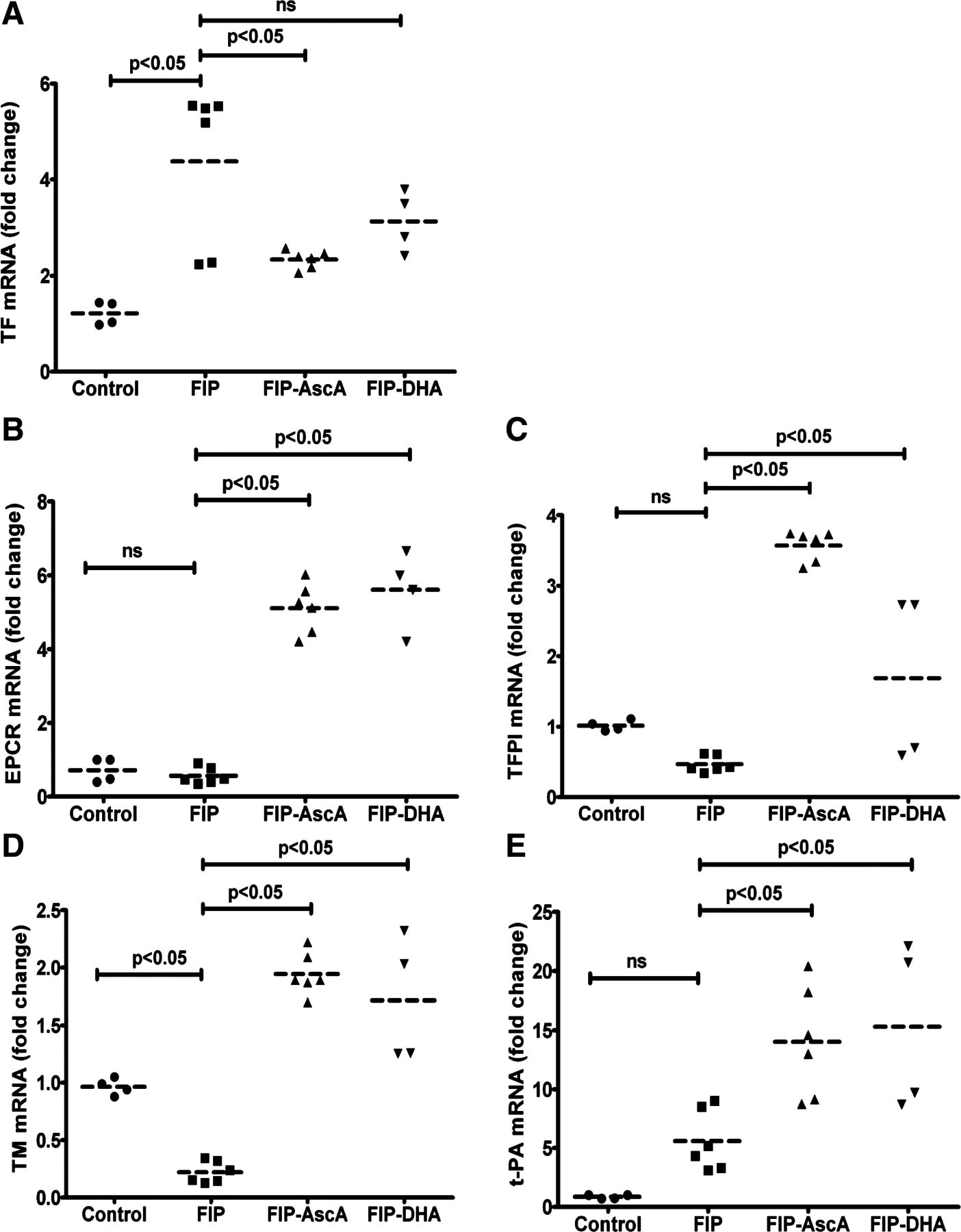
Bacterial infections of the lungs and abdomen are among the most common causes of sepsis. Abdominal peritonitis often results in acute lung injury (ALI). Recent reports demonstrate a potential benefit of parenteral vitamin C [ascorbic acid (AscA)] in the pathogenesis of sepsis. Therefore we examined the mechanisms of vitamin C supplementation in the setting of abdominal peritonitis-mediated ALI. We hypothesized that vitamin C supplementation would protect lungs by restoring alveolar epithelial barrier integrity and preventing sepsis-associated coagulopathy. Male C57BL/6 mice were intraperitoneally injected with a fecal stem solution to induce abdominal peritonitis (FIP) 30 min prior to receiving either AscA (200 mg/kg) or dehydroascorbic acid (200 mg/kg). Variables examined included survival, extent of ALI, pulmonary inflammatory markers (myeloperoxidase, chemokines), bronchoalveolar epithelial permeability, alveolar fluid clearance, epithelial ion channel, and pump expression (aquaporin 5, cystic fibrosis transmembrane conductance regulator, epithelial sodium channel, and Na+-K+-ATPase), tight junction protein expression (claudins, occludins, zona occludens), cytoskeletal rearrangements (F-actin polymerization), and coagulation parameters (thromboelastography, pro- and anticoagulants, fibrinolysis mediators) of septic blood. FIP-mediated ALI was characterized by compromised lung epithelial permeability, reduced alveolar fluid clearance, pulmonary inflammation and neutrophil sequestration, coagulation abnormalities, and increased mortality. Parenteral vitamin C infusion protected mice from the deleterious consequences of sepsis by multiple mechanisms, including attenuation of the proinflammatory response, enhancement of epithelial barrier function, increasing alveolar fluid clearance, and prevention of sepsis-associated coagulation abnormalities. Parenteral vitamin C may potentially have a role in the management of sepsis and ALI associated with sepsis.
3. Epithelial-Mesenchymal TransitionにおけるTwist & Smail
Epithelial-Mesenchymal Transition (EMT;上皮間葉移行) は1980年代初めにElizabeth Hayらが提唱した上皮細胞が間葉系様細胞に形態変化する現象であり,初期胚発生における原腸陥入,神経提細胞の運動や器官形成過程特に,心臓や腎臓また口蓋形成での重要性がこれまでに明らかとなっている1。一方,EMTの獲得が運動性の亢進や細胞外基質の蓄積をもたらすことから,癌細胞の浸潤や線維症との関連も示唆されている。TwistはbHLH型転写因子で,myogenin プロモーター上でE12とヘテロ2量体を形成し,遺伝子発現を抑制し,さらにSeathre-Chotzen syndromeの原因遺伝子として知られている。Twistの効果は,細胞特異性や他の補助的分子の存在が必要となる可能性が示唆されている。
Koji Sakamoto, Naozumi Hashimoto, Yasuhiro Kondoh, Kazuyoshi Imaizumi, Daisuke Aoyama, Takashi Kohnoh, Masaaki Kusunose, Motohiro Kimura, Tsutomu Kawabe, Hiroyuki Taniguchi, and Yoshinori Hasegawa
Differential modulation of surfactant protein D under acute and persistent hypoxia in acute lung injury
Am J Physiol Lung Cell Mol Physiol July 1, 2012 303:L43-L53
Hypoxia contributes to the development of fibrosis with epithelial-mesenchymal transition (EMT) via stimulation of hypoxia-inducible factor 1α (HIF-1α) and de novo twist expression. Although hypoxemia is associated with increasing levels of surfactant protein D (SP-D) in acute lung injury (ALI), the longitudinal effects of hypoxia on SP-D expression in lung tissue injury/fibrosis have not been fully evaluated. Here, the involvement of hypoxia and SP-D modulation was evaluated in a model of bleomycin-induced lung injury. We also investigated the molecular mechanisms by which hypoxia might modulate SP-D expression in alveolar cells, by using a doxycycline (Dox)-dependent HIF-1α expression system. Tissue hypoxia and altered SP-D levels were present in bleomycin-induced fibrotic lesions. Acute hypoxia induced SP-D expression, supported by the finding that Dox-induced expression of HIF-1α increased SP-D expression. In contrast, persistent hypoxia repressed SP-D expression coupled with an EMT phenotype and twist expression. Long-term expression of HIF-1α caused SP-D repression with twist expression. Ectopic twist expression repressed SP-D expression. The longitudinal observation of hypoxia and SP-D levels in ALI in vivo was supported by the finding that HIF-1α expression stabilized by acute hypoxia induced increasing SP-D expression in alveolar cells, whereas persistent hypoxia induced de novo twist expression in these cells, causing repression of SP-D and acquisition of an EMT phenotype. Thus this is the first study to demonstrate the molecular mechanisms, in which SP-D expression under acute and persistent hypoxia in acute lung injury might be differentially modulated by stabilized HIF-1α expression and de novo twist expression.
4. Ovalbumin 誘導喘息モデル
Kimitake Tsuchiya, Sana Siddiqui, Paul-André Risse, Nobuaki Hirota, and James G. Martin
The presence of LPS in OVA inhalations affects airway inflammation and AHR but not remodeling in a rodent model of asthma
Am J Physiol Lung Cell Mol Physiol July 1, 2012 303:L54-L63
Ovalbumin (OVA) is the most frequently used allergen in animal models of asthma. Lipopolysaccharide (LPS) contaminating commercial OVA may modulate the evoked airway inflammatory response to OVA. However, the effect of LPS in OVA on airway remodeling, especially airway smooth muscle (ASM) has not been evaluated. We hypothesized that LPS in commercial OVA may enhance allergen-induced airway inflammation and remodeling. Brown Norway rats were sensitized with OVA on day 0. PBS, OVA, or endotoxin-free OVA (Ef-OVA) was instilled intratracheally on days 14, 19, 24. Bronchoalveolar lavage (BAL) fluid, lung, and intrathoracic lymph node tissues were collected 48 h after the last challenge. Immunohistochemistry for α-smooth muscle actin, Periodic-Acid-Schiff staining, and real-time qPCR were performed. Airway hyperresponsiveness (AHR) was also measured. BAL fluid macrophages, eosinophils, neutrophils, and lymphocytes were increased in OVA-challenged animals, and macrophages and neutrophils were significantly lower in Ef-OVA-challenged animals. The ASM area in larger airways was significantly increased in both OVA and Ef-OVA compared with PBS-challenged animals. The mRNA expression of IFN-γ and IL-13 in lung tissues and IL-4 in lymph nodes was significantly increased by both OVA and Ef-OVA compared with PBS and were not significantly different between OVA and Ef-OVA. Monocyte chemoattractant protein (MCP)-1 in BAL fluid and AHR were significantly increased in OVA but not in Ef-OVA. LPS contamination in OVA contributes to the influx of macrophages and MCP-1 increase in the airways and to AHR after OVA challenges but does not affect OVA-induced Th1 and Th2 cytokine expression, goblet cell hyperplasia, and ASM remodeling.
5. 肺高血圧におけるglucose-6-phosphate dehydrogenaseとPKG
Sukrutha Chettimada, Dhwajbahadur K. Rawat, Nupur Dey, Robert Kobelja, Zachary Simms, Michael S. Wolin, Thomas M. Lincoln, and Sachin A. Gupte
Glc-6-PD and PKG contribute to hypoxia-induced decrease in smooth muscle cell contractile phenotype proteins in pulmonary artery
Am J Physiol Lung Cell Mol Physiol July 1, 2012 303:L64-L74
Persistent hypoxic pulmonary vasoconstriction (HPV) plays a significant role in the pathogenesis of pulmonary hypertension, which is an emerging clinical problem around the world. We recently showed that hypoxia-induced activation of glucose-6-phosphate dehydrogenase (Glc-6-PD) in pulmonary artery smooth muscle links metabolic changes within smooth muscle cells to HPV and that inhibition of Glc-6PD reduces acute HPV. Here, we demonstrate that exposing pulmonary arterial rings to hypoxia (20–30 Torr) for 12 h in vitro significantly (P < 0.05) reduces (by 30–50%) SM22α and smooth muscle myosin heavy chain expression and evokes HPV. Glc-6-PD activity was also elevated in hypoxic pulmonary arteries. Inhibition of Glc-6-PD activity prevented the hypoxia-induced reduction in SM22α expression and inhibited HPV by 80–90% (P < 0.05). Furthermore, Glc-6-PD and protein kinase G (PKG) formed a complex in pulmonary artery, and Glc-6-PD inhibition increased PKG-mediated phosphorylation of VASP (p-VASP). In turn, increasing PKG activity upregulated SM22α expression and attenuated HPV evoked by Glc-6-PD inhibition. Increasing passive tension (from 0.8 to 3.0 g) in hypoxic arteries for 12 h reduced Glc-6-PD, increased p-VASP and SM22α levels, and inhibited HPV. The present findings indicate that increases in Glc-6-PD activity influence PKG activity and smooth muscle cell phenotype proteins, all of which affect pulmonary artery contractility and remodeling.
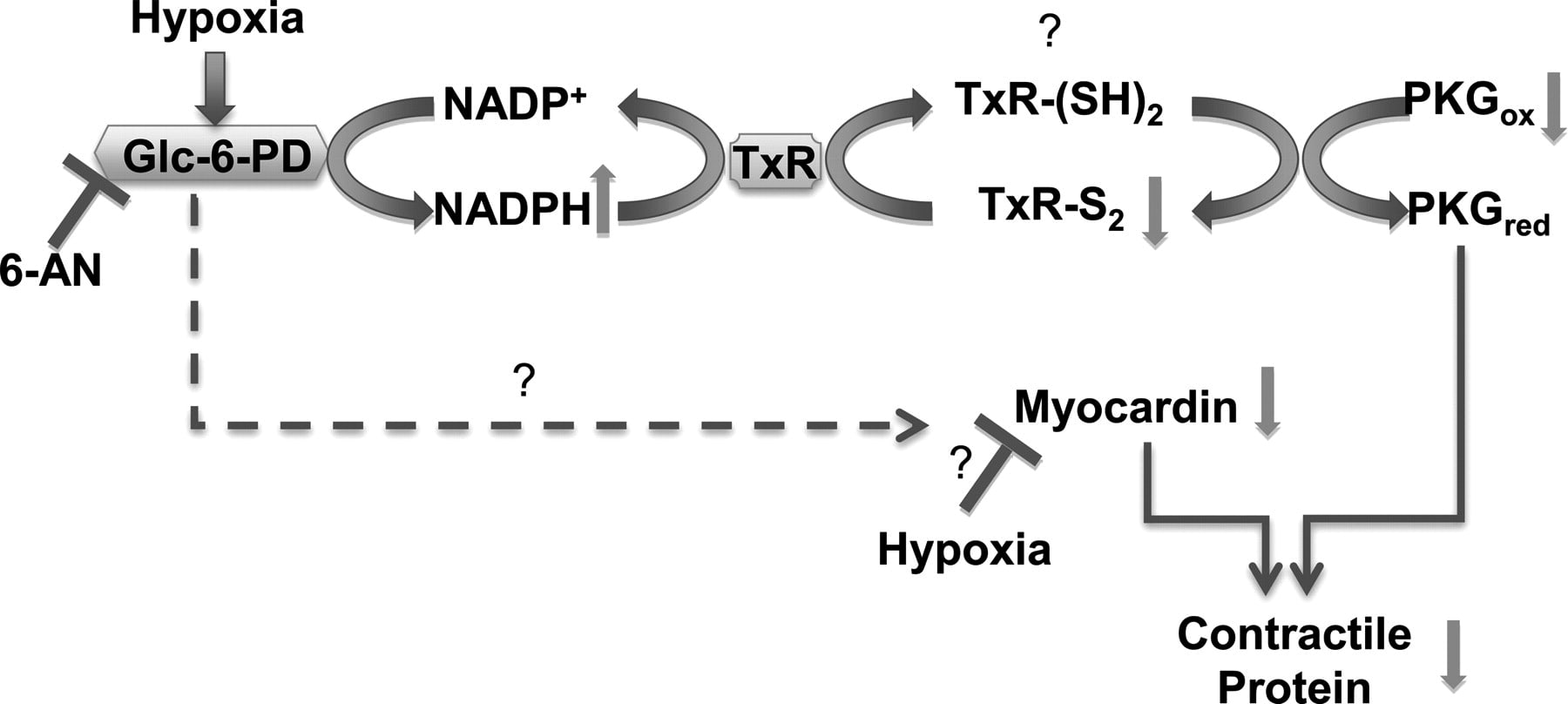
Glc-6-PD and thioredoxin reductase-1 (TxR-1) form a complex with PKG and regulate hypoxia-evoked changes in the expression of contractile proteins in pulmonary artery. Schematic illustrating a potential pathway through which hypoxia affects activity of Glc-6-PD and PKG and expression of contractile proteins. Inhibition of Glc-6-PD-derived NADPH redox reduces TxR-1 activity, which oxidizes thiols on PKG and activates it. Increase in PKG activity reexpresses contractile proteins, whereas myocardin expression is increased in a PKG-independent manner.
最新の画像[もっと見る]
-
 お知らせ 世界敗血症デー 京都 2024
2ヶ月前
お知らせ 世界敗血症デー 京都 2024
2ヶ月前
-
 お知らせ 世界敗血症デー 京都 2024
2ヶ月前
お知らせ 世界敗血症デー 京都 2024
2ヶ月前
-
 お知らせ 世界敗血症デー 京都 2024
2ヶ月前
お知らせ 世界敗血症デー 京都 2024
2ヶ月前
-
 お知らせ 世界敗血症デー 京都 2024
2ヶ月前
お知らせ 世界敗血症デー 京都 2024
2ヶ月前
-
 お知らせ 世界敗血症デー 京都 2024
2ヶ月前
お知らせ 世界敗血症デー 京都 2024
2ヶ月前
-
 セミナーのお知らせ 多発外傷カンファレンス with デューク大学
3ヶ月前
セミナーのお知らせ 多発外傷カンファレンス with デューク大学
3ヶ月前
-
 執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
3ヶ月前
執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
3ヶ月前
-
 執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
3ヶ月前
執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
3ヶ月前
-
 執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
3ヶ月前
執筆紹介 麻酔科学と救急医学 ー場の成長と発展のためにー 救急医療 人道の道 JAPAN and the World
3ヶ月前
-
 救急・集中治療 人食いバクテリアの診断と治療
8ヶ月前
救急・集中治療 人食いバクテリアの診断と治療
8ヶ月前
「ジャーナルクラブ 松田直之指導」カテゴリの最新記事
 ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年12月号 Sonic Hed...
ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年12月号 Sonic Hed... 2012年12月13日 基盤ジャーナルクラブ 心血管基礎研究関連
2012年12月13日 基盤ジャーナルクラブ 心血管基礎研究関連 文献紹介 Hypoglycemia and Risk of Death in Critically Ill Patients N Engl J...
文献紹介 Hypoglycemia and Risk of Death in Critically Ill Patients N Engl J... ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年11月号
ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年11月号 ジャーナルクラブ Circulation Res 2012, September 14 & 28
ジャーナルクラブ Circulation Res 2012, September 14 & 28 ジャーナルクラブ British J Pharmacol 2012. October Volume 167, Issue 3
ジャーナルクラブ British J Pharmacol 2012. October Volume 167, Issue 3 ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年9月号
ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年9月号 ジャーナルクラブ Circulation Research 2012. August
ジャーナルクラブ Circulation Research 2012. August ジャーナルクラブ British Journal of Pharmacology 2012. September
ジャーナルクラブ British Journal of Pharmacology 2012. September ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年7月号
ジャーナルクラブ Am J Physiol Lung Cell Mol Physiol 2012年7月号

















