Lithium-ion battery (Wikipedia)
"The three primary functional components of a lithium-ion battery are the positive and negative electrodes and electrolyte. Generally, the negative electrode of a conventional lithium-ion cell is made from(から成る、作られる)carbon. The positive electrode is(である、から成る)a metal oxide, and the electrolyte is a lithium salt in an organic solvent.[45] The electrochemical roles of the electrodes reverse(逆になる)between anode and cathode, depending on the direction of current flow through the cell.
The most commercially popular negative electrode is graphite. The positive electrode is generally one of three materials: a layered oxide (such as lithium cobalt oxide), a polyanion (such as lithium iron phosphate) or a spinel (such as lithium manganese oxide).[46]
The electrolyte is typically a mixture of organic carbonates such as ethylene carbonate or diethyl carbonate containing complexes of lithium ions.[47] These non-aqueous electrolytes generally use non-coordinating(非配位性)anion salts such as lithium hexafluorophosphate (LiPF
6), lithium hexafluoroarsenate monohydrate (LiAsF
6), lithium perchlorate (LiClO
4), lithium tetrafluoroborate (LiBF
4) and lithium triflate (LiCF
3SO
3)."
"The participants(関与する)in the electrochemical reactions in a lithium-ion battery are the negative and positive electrodes with the electrolyte providing a conductive medium for Lithium-ions to move between the electrodes.
Both electrodes allow lithium ions to move in and out of their interiors. During insertion (or intercalation)(挿入)ions move into the electrode. During the reverse process, extraction (or deintercalation)(脱離), ions move back out. When a lithium-ion based cell is discharging(放電している), the positive Lithium ion moves from the negative electrode (usually graphite = " " below) and enters the positive electrode (lithium containing compound). When the cell is charging(充電している; cf. being charged), the reverse occurs.
" below) and enters the positive electrode (lithium containing compound). When the cell is charging(充電している; cf. being charged), the reverse occurs.
Useful work is performed when electrons flow through a closed external circuit. The following equations show one example of the chemistry, in units(単位)of moles, making it possible to use coefficient 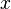 .
.
The cathode (marked +) half-reaction is:[54]

The anode (marked -) half reaction is:

The overall reaction has its limits. Overdischarge(過放電)supersaturates lithium cobalt oxide, leading to the production(生成、発生、生じる) of lithium oxide,[55] possibly by the following irreversible reaction:

Overcharge(過充電)up to 5.2 volts leads to the synthesis of cobalt(IV) oxide, as evidenced by x-ray diffraction:[56]
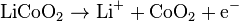
In a lithium-ion battery the lithium ions are transported to and from the positive or negative electrodes by oxidizing the transition metal, cobalt (Co)(遷移金属、すなわちコバルト), in Li
1-xCoO
2 from Co3+
to Co4+
during charge, and reduced from Co4+
to Co3+
during discharge. The cobalt electrode reaction is only reversible for x < 0.5, limiting the depth of discharge allowable. This chemistry was used in the Li-ion cells developed by Sony in 1990."
"Electrolytes[edit]
The cell voltages given in the Electrochemistry section are larger than the potential at which aqueous solutions will electrolyze(電解する).
Liquid electrolytes(液体電解質)in lithium-ion batteries consist of(から成る)lithium salts, such as LiPF
6, LiBF
4 or LiClO
4 in an organic solvent, such as ethylene carbonate, dimethyl carbonate, and diethyl carbonate.[57] A liquid electrolyte acts as(作用、機能する)a conductive pathway for the movement of cations passing from the positive to the negative electrodes during discharge(放電中). Typical conductivities(複数形)of liquid electrolyte at room temperature (20 °C (68 °F)) are in the range of 10 mS/cm, increasing by approximately 30–40% at 40 °C (104 °F) and decreasing slightly at 0 °C (32 °F).[58]
The combination of linear and cyclic carbonates (e.g., ethylene carbonate (EC) and dimethyl carbonate (DMC)) offers(により得ることができる)high conductivity and SEI-forming ability. A mixture of a high ionic conductivity and low viscosity carbonate solvents is needed, because the two properties are mutually exclusive in a single material.[59]
Organic solvents easily decompose(分解する)on the negative electrodes during charge(充電中). When appropriate organic solvents are used as the electrolyte, the solvent decomposes on initial charging and forms a solid layer called the solid electrolyte interphase (SEI),[60] which is electrically insulating yet provides significant ionic conductivity. The interphase prevents further decomposition of the electrolyte after the second charge. For example, ethylene carbonate is decomposed at a relatively high voltage, 0.7 V vs. lithium, and forms a dense and stable interface.[61]
Composite electrolytes based on POE (poly(oxyethylene)) provide(が得られる)a relatively stable interface.[62][63] It can be either solid (high molecular weight) and be applied in dry Li-polymer cells, or liquid (low molecular weight) and be applied in regular Li-ion cells.
Room temperature ionic liquids (RTILs) are another approach to(するための方法、手段)limiting the flammability and volatility of organic electrolytes.[64]"
"Materials[edit]
The increasing demand for batteries has led vendors and academics to focus on improving the energy density, operating temperature, safety, durability, charging time, output power, and cost of lithium ion battery solutions. The following materials have been used in commercially available cells. Research into other materials continues.
Cathode materials are generally constructed out of(から成る、構成される)two general materials: LiCoO¬2 and LiMn2O4. The cobalt-based material develops a pseudo tetrahedral structure that allows for two-dimensional Lithium ion diffusion.[78] The cobalt-based cathodes are ideal due to their high theoretical specific heat capacity, high volumetric capacity, low self-discharge, high discharge voltage, and good(良好)cycling performance. Limitations include the high cost of the material, slight toxicity, and low thermal stability.[79] The manganese-based materials adopt a cubic crystal lattice system, which allows for three-dimensional Lithium ion diffusion.[78] Manganese cathodes are attractive(興味深い、注目されている)because manganese is cheaper and less toxic than other materials used. Limitations include the tendency for manganese to dissolve into the electrolyte during cycling leading to poor cycling stability for the cathode.[79] Cobalt-based cathodes are the most common however other materials are beginning to be developed to make cheaper and less toxic cathodes.[80]"



























※コメント投稿者のブログIDはブログ作成者のみに通知されます