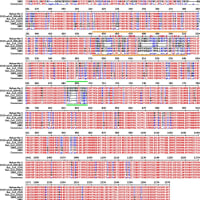
Reactions (Pa-Pb) εo (V vs. SHE) Ref.
Pa3+ + 3e- ↔ Pa -1.34 1
Pa4+ + 4e- ↔ Pa -1.49 1
Pa4+ + e- ↔ Pa3+ -1.9 1
Pb2+ + 2e- ↔ Pb -0.1262(1), -0.1263(2)
Pb2+ + 2e- ↔ Pb(Hg) -0.1205 1
Pb4+ + 2e- ↔ Pb2+ 1.8 1
PbBr2 + 2e- ↔ Pb + 2Br- -0.284(1), -0.280(2)
PbCO3 + 2e- ↔ Pb + CO32- -0.509 2
PbCl2 + 2e- ↔ Pb + 2Cl- -0.2675(1), -0.268(2)
PbF2 + 2e- ↔ Pb + 2F- -0.3444 1
PbI2 + 2e- ↔ Pb + 2I- -0.365 1, 2
PbO(red) + H2O + 2e- ↔ Pb + 2OH- -0.580(1, 2a), -0.579(2b)
PbO2(α) + H2O + 2e- ↔ PbO(red) + 2OH- 0.247(1), 0.249(2)
PbO2(α) + 4H+ + 2e- ↔ Pb2+ + 2H2O 1.455 (1), 1.468(2)
PbO2(α) + 4H+ + SO42- + 2e- ↔ PbSO4 + 2H2O 1.6913(1), 1.698(2)
HPbO2- + H2O + 2e- ↔ Pb+ 3OH- -0.537 1
PbHPO4 + 2e- ↔ Pb + HPO42- -0.465 1
Pb3(PO4)2 + 6e- ↔ 3Pb + 2PO43- -0.557 2
PbS + 2e- ↔ Pb + S2- -0.954 2
PbSO4 + 2e- ↔ Pb + SO42- -0.3588 1
PbSO4 + 2e- ↔ Pb(Hg) + SO42- -0.3505 1, 2