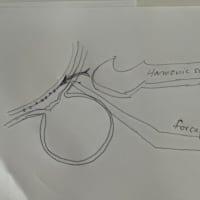
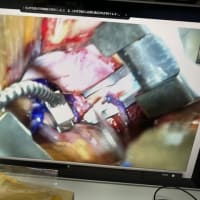
ランセットに発表された論文によると、三尖弁逆流に対するカテーテル治療が試みられており、これによる有効性が十分証明されています。僧帽弁手術に同時に右房も切開して三尖弁輪の縫縮を行うことは、三尖弁逆流のある患者さんにとって予後を改善する、という趣旨で弁輪を作成している業者が宣伝していますが、今後は同時に右房を開けなくともカテーテル治療とハイブリッドで三尖弁逆流を制御する時代になるかもしれません。
Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study
Summary
Background
Tricuspid regurgitation is a prevalent disease associated with high morbidity and mortality, with few treatment options. The aim of the TRILUMINATE trial is to evaluate the safety and effectiveness of TriClip, a minimally invasive transcatheter tricuspid valve repair system, for reducing tricuspid regurgitation.
Methods
The TRILUMINATE trial is a prospective, multicentre, single-arm study in 21 sites in Europe and the USA. Patients with moderate or greater triscuspid regurgitation, New York Heart Association class II or higher, and who were adequately treated per applicable standards were eligible for enrolment. Patients were excluded if they had systolic pulmonary artery pressure of more than 60 mm Hg, a previous tricuspid valve procedure, or a cardiovascular implantable electronic device that would inhibit TriClip placement. Participants were treated using a clip-based edge-to-edge repair technique with the TriClip tricuspid valve repair system. Tricuspid regurgitation was graded using a five-class grading scheme (mild, moderate, severe, massive, and torrential) that expanded on the standard American Society of Echocardiography grading scheme. The primary efficacy endpoint was a reduction in tricuspid regurgitation severity by at least one grade at 30 days post procedure, with a performance goal of 35%, analysed in all patients who had an attempted tricuspid valve repair procedure upon femoral vein puncture. The primary safety endpoint was a composite of major adverse events at 6 months, with a performance goal of 39%. Patients were excluded from the primary safety analysis if they did not reach 6-month follow-up and did not have a major adverse event during previous follow-ups. The trial has completed enrolment and follow-up is ongoing; it is registered with ClinicalTrials.gov , number NCT03227757 .
Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study
Summary
Background
Tricuspid regurgitation is a prevalent disease associated with high morbidity and mortality, with few treatment options. The aim of the TRILUMINATE trial is to evaluate the safety and effectiveness of TriClip, a minimally invasive transcatheter tricuspid valve repair system, for reducing tricuspid regurgitation.
Methods
The TRILUMINATE trial is a prospective, multicentre, single-arm study in 21 sites in Europe and the USA. Patients with moderate or greater triscuspid regurgitation, New York Heart Association class II or higher, and who were adequately treated per applicable standards were eligible for enrolment. Patients were excluded if they had systolic pulmonary artery pressure of more than 60 mm Hg, a previous tricuspid valve procedure, or a cardiovascular implantable electronic device that would inhibit TriClip placement. Participants were treated using a clip-based edge-to-edge repair technique with the TriClip tricuspid valve repair system. Tricuspid regurgitation was graded using a five-class grading scheme (mild, moderate, severe, massive, and torrential) that expanded on the standard American Society of Echocardiography grading scheme. The primary efficacy endpoint was a reduction in tricuspid regurgitation severity by at least one grade at 30 days post procedure, with a performance goal of 35%, analysed in all patients who had an attempted tricuspid valve repair procedure upon femoral vein puncture. The primary safety endpoint was a composite of major adverse events at 6 months, with a performance goal of 39%. Patients were excluded from the primary safety analysis if they did not reach 6-month follow-up and did not have a major adverse event during previous follow-ups. The trial has completed enrolment and follow-up is ongoing; it is registered with ClinicalTrials.gov , number NCT03227757 .