厚生労働科学研究成果データベース
http://mhlw-grants.niph.go.jp/niph/search/NIST00.do
200618009A0001.pdf (平成18年度総括研究報告書)
200618009B0001.pdf (平成18年度総合研究報告書)
研究成果
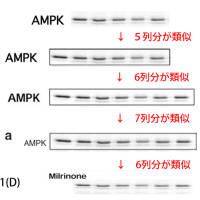
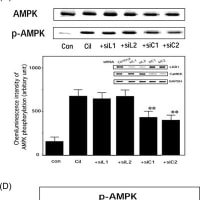
上記論文#15のFigure 2と、論文#24のFigure 3Aが、同一データである。
上記論文#15のFigure 3と、論文#24のFigure 3が、同一データである。
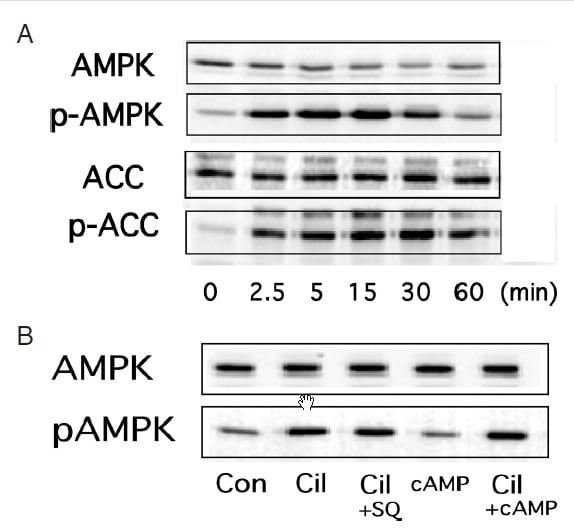
↓論文#3のFigure 2
Journal of Atherosclerosis and Thrombosis
Vol. 17 (2010) , No. 5 503-509
Copyright (c) 2010 Japan Atherosclerosis Society
(A) Cilostazol-mediated activation of AMPK in rat VSMC. VSMC were treated with colostazol (100μM) for the indicated time periods before lysis, after which samples of cell lysate were probed with antibodies specific for the phosphorylated forms of AMPK and acetyl-CoA carboxylase (ACC). (B) HUVEC treated with cilostazol (100 μM) alone or in the presence of an adenylate cyclase inhibitor SQ22536 (10 μM) or a cell-permeable cAMP analog pCTP-cAMP (100 μM). After 15 min of incubation, the cells were lysed and p-AMPK activity was analyzed. Three independent studies showed similar results.
↓論文#4のFigure 1(A)(画像クリックで解像度の高い画像が表示されます。)

Figure 1
(A) Cilostazol activates AMP-activated protein kinase (AMPK) in vascular endothelial cells. Human umbilical vein endothelial cells (HUVEC) were treated with cilostazol (100 μM) for the indicated time periods before lysis, after which each cell lysate sample was probed with antibodies specific for phosphorylated forms of AMPK and acetyl-CoA carboxylase (ACC).
Hattori Y et al. Cardiovasc Res 2009;81:133-139
Copyright the European Society of Cardiology.
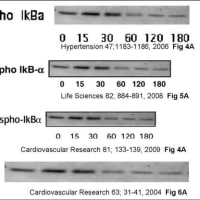
↓論文#4のFigure 4

Hattori Y et al. Cardiovasc Res 2009;81:133-139
Copyright the European Society of Cardiology.
Figure 4
(A) Human umbilical vein endothelial cells (HUVEC) were incubated with tumour necrosis factor alpha (TNFα) for 0–180 min. The cells were lysed and subjected to western blot analysis using anti-IκB-α and anti-phospho-IκB-α antibodies. (B) The effect of cilostazol on IκB-α degradation in HUVEC. Cells were incubated for 30 min with cilostazol (30 and 100 μM), followed by TNFα for 30 min. Cells were then lysed and subjected to western blot analysis using anti-IκBα antibody. (C) The effect of cilostazol on IKK activity in HUVEC. Cells were incubated for 30 min with cilostazol (30 and 100 μM), followed by TNFα for 15 min. Cells were then lysed and immunoprecipitated with anti-IKKα/β antibody and used for kinase assay using recombinant IκBα as a substrate. Note that equal band densities for IKKα/β and GST-IκBα were observed. Three independent studies showed similar results.
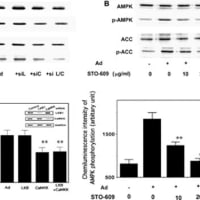
↓論文#5のFigure 3(B)

Figure 3 (b) Effect of the AMPK inhibitor compound C (CC) on
the telmisartan-mediated inhibition of TNFa-induced NF-kB activation.
Inset: Time course analysis for pAMPK by telmisartan or metformin. Data
represent the means±s.d. (n¼4). **Po0.01 compared with the control
value (TNFa only). NS, not significant between the absence and presence
of CC.
Copyright 2009 The Japanese Society of Hypertension
Hypertension Research (2009) 32, 765–769
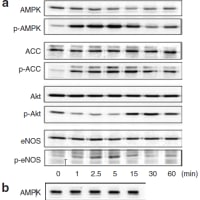
↓論文#8のFigure 1(A)(B)

Fig. 1. (A) Fenofibrate activates AMPK in vascular endothelial cells. HUVEC were treated with 100 μmol/L fenofibrate for the indicated time periods before lysis, after which cell lysates were probed with antibodies specific for AMPK, ACC, Akt, or eNOS, or their phosphorylated forms. (B) WY14643 (100 μmol/L) activates AMPK in HUVEC.
↓論文#8のFigure 5

Fig. 5. (A) HUVEC were incubated with TNFα for 0–180 min. The cells were lysed and subjected to Western blot analysis using anti-IκB-α and anti-phospho-IκB-α antibodies. (B) The effect of fenofibrate on IκB-α degradation in HUVEC. Cells were incubated for 30 min with fenofibrate (30 and 100 μM), followed by TNFα for 30 min. Cells were then lysed and subjected to Western blot analysis using anti-IκB-α antibody. (C) The effect of fenofibrate on IKK activity in HUVEC. Cells were incubated for 30 min with metformin (30 and 100 μM), followed by TNFα for 15 min. Cells were then lysed and immunoprecipitated with anti-IKKα/β antibody and used for kinase assay using recombinant IκB-α as a substrate. Note that equal band densities for IKKα/β- and IκB-α were observed. Three independent experiments for (A) and (B) and two independent experiments for (C) were performed. Similar results were obtained in each experiment and representative photos were shown.
↓論文#9のFigure 1(A)

Figure 1 | Cilostazol activates AMP-activated protein kinase (AMPK) in
vascular endothelial cells. (a) human umbilical vein endothelial cells (HUVECs)
were treated with 100 μmol/l cilostazol for the indicated time periods before
lysis, after which cell lysates were probed with antibodies specific for AMPK,
acetyl-CoA carboxylase (ACC), Akt, or endothelial nitric oxide synthase
(eNOS), or their phosphorylated forms.
↓論文#14のFigure 3 (画像クリックで解像度の高い画像が表示されます。)

Hattori, Y. et al. Hypertension 2006;47:1183-1188
Copyright ©2006 American Heart Association
Figure 3. (A) HUVECs were incubated with TNF- for 0 to 180 minutes. The cells were lysed and subjected to Western blot analysis using anti-IkB- and anti-phospho-IkB- antibodies. (B) The effect of metformin on IB-degradation in HUVECs. Cells were incubated for 30 minutes with metformin (3 and 10 mmol/L), followed by TNF- for 30 minutes. Cells were then lysed and subjected to Western blot analysis using anti-IkB- antibody. (C) The effect of metformin on IKK activity in HUVECs. Cells were incubated for 30 minutes with metformin (3 and 10 mmol/L), followed by TNF- for 15 minutes. Cells were then lysed and immunoprecipitated with anti-IKK/ß antibody and used for kinase assay using recombinant IkB- as a substrate. Note that equal band densities for IKK/ß and IkB- were observed.
↓論文#21のFigure 6(画像をクリックすると解像度の高い画像が表示されます。)

Hattori Y et al. Cardiovasc Res 2004;63:31-40
Copyright © 2004, European Society of Cardiology
Fig. 6
(A) Effect of NOR3 and SIN-1 on degradation and phosphorylation of I B-α in rat VSMC. Cells were incubated for 45 min with various concentrations of NOR3 (1 mM) or SIN-1 (1 mM), followed by LPS (30 μg/ml) for 0–180 min. Cell were lysed and subjected to Western blot analysis using anti-I B-α antibody and anti-phospho-I B-α. (B) Effect of NOR3 and SIN-1 on IKK activity in rat VSMC. Cells were incubated for 45 min with various concentrations of NOR3 (0.25–1 mM) or SIN-1 (1 mM), followed by LPS (30 μg/ml) for 15 min. Cells were lysed and immunoprecipitated with anti-IKKα/β antibody and used for kinase assay using recombinant I B-α as substrate. Note that equal intensities of IKKα/β- and I B-α-specific bands are obtained.
http://mhlw-grants.niph.go.jp/niph/search/NIST00.do
200618009A0001.pdf (平成18年度総括研究報告書)
200618009B0001.pdf (平成18年度総合研究報告書)
| 文献番号 | 200618009A |
| 研究課題 | 各種高脂血症治療薬の糖尿病性心血管病進展予防効果の総合的検討(若手医師・協力者活用に要する研究) |
| 研究年度 | 平成18(2006)年度 |
| 主任研究者(所属機関) | 服部 良之(独協医科大学内分泌代謝内科) |
| 研究区分 | 厚生労働科学研究費補助金 厚生科学基盤研究分野 臨床研究基盤整備推進研究 |
| 開始年度 | 平成16(2004)年度 |
| 終了予定年度 | 平成18(2006)年度 |
| 研究費 | 4,140,000円 |
研究成果
上記論文#15のFigure 2と、論文#24のFigure 3Aが、同一データである。
上記論文#15のFigure 3と、論文#24のFigure 3が、同一データである。
論文#4のFigure 4(A)のPhospho-IkBαの画像と、
論文#8のFigure 5(A)のPhospho-IkB-αの画像と、
上記論文#14のFigure 3(A)のPhospho-IkBaの画像と、
論文#21のFigure 6(A)のPhospho IκB-αの画像の、
4つの画像は全て同一画像であり、データの捏造(流用)が疑われる。
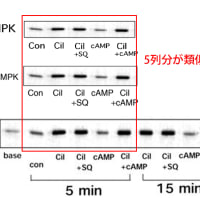
さらに、この上記論文#21のFigure 6(A)の上から2段目のPhospho IκB-αの画像の右3列分(左から4列目、5列目、6列目)の画像は、
同論文のFigure 6(A)の最下段のPhospho IkB-αの画像の左3列分(左から1列目、2列目、3列目)の画像と同一であり、データの捏造(流用)が疑われる。
また、
論文#4のFigure 4(B)のIkBαの画像と、
論文#8のFigure 5(B)のIkB-αの画像と、
上記論文#14のFigure 3(B)のIkBaの画像と、
論文#21のFigure 6(A)のONOO- IκB-αの画像の左4列の画像の、
4つの画像は全て同一画像であり、データの捏造(流用)が疑われる。
論文#8のFigure 5(A)のPhospho-IkB-αの画像と、
上記論文#14のFigure 3(A)のPhospho-IkBaの画像と、
論文#21のFigure 6(A)のPhospho IκB-αの画像の、
4つの画像は全て同一画像であり、データの捏造(流用)が疑われる。
さらに、この上記論文#21のFigure 6(A)の上から2段目のPhospho IκB-αの画像の右3列分(左から4列目、5列目、6列目)の画像は、
同論文のFigure 6(A)の最下段のPhospho IkB-αの画像の左3列分(左から1列目、2列目、3列目)の画像と同一であり、データの捏造(流用)が疑われる。
また、
論文#4のFigure 4(B)のIkBαの画像と、
論文#8のFigure 5(B)のIkB-αの画像と、
上記論文#14のFigure 3(B)のIkBaの画像と、
論文#21のFigure 6(A)のONOO- IκB-αの画像の左4列の画像の、
4つの画像は全て同一画像であり、データの捏造(流用)が疑われる。
また、
上記論文#14のFigure 3(C)の最上段のIkB-aの画像の右3列分(左から2列目、3列目、4列目)の画像と、
論文#21のFigure 6(B)の最上段のIκB-αの画像の中央3列分(左から2列目、3列目、4列目)の画像とが、
同一画像である。
さらに、この上記論文#21のFigure 6(B)の最上段のIkB-αの画像の左から2列目のバンド画像は、
論文#21のFigure 6(B)の下から3段目のIκB-αの画像の左から2列目のバンド画像と、
同一画像であり、画像の流用(データ捏造)が疑われる。
また、
上記論文#14のFigure 3(C)のIKKa/bの画像の左3列分(左から1列目、2列目、3列目)の画像と
論文#21のFigure 6(B)の下から5段目のIKKα/βの画像の左3列分(左から1列目、2列目、3列目)の画像と、
論文#21のFigure 6(B)の下から2段目のIKKα/βの画像が、
同一画像であり、画像の流用(データ捏造)が疑われる。
また、
上記論文#14のFigure 3(C)の最下段のIkB-aの画像の左3列分(左から1列目、2列目、3列目)の画像と、
論文#21のFigure 6(B)の下から4段目のIκB-αの画像の左3列分(左から1列目、2列目、3列目)の画像と、
論文#21のFigure 6(B)の最下段のIκB-αの画像が、
同一画像であり、画像の流用(データ捏造)が疑われる。
上記論文#14のFigure 3(C)の最上段のIkB-aの画像の右3列分(左から2列目、3列目、4列目)の画像と、
論文#21のFigure 6(B)の最上段のIκB-αの画像の中央3列分(左から2列目、3列目、4列目)の画像とが、
同一画像である。
さらに、この上記論文#21のFigure 6(B)の最上段のIkB-αの画像の左から2列目のバンド画像は、
論文#21のFigure 6(B)の下から3段目のIκB-αの画像の左から2列目のバンド画像と、
同一画像であり、画像の流用(データ捏造)が疑われる。
また、
上記論文#14のFigure 3(C)のIKKa/bの画像の左3列分(左から1列目、2列目、3列目)の画像と
論文#21のFigure 6(B)の下から5段目のIKKα/βの画像の左3列分(左から1列目、2列目、3列目)の画像と、
論文#21のFigure 6(B)の下から2段目のIKKα/βの画像が、
同一画像であり、画像の流用(データ捏造)が疑われる。
また、
上記論文#14のFigure 3(C)の最下段のIkB-aの画像の左3列分(左から1列目、2列目、3列目)の画像と、
論文#21のFigure 6(B)の下から4段目のIκB-αの画像の左3列分(左から1列目、2列目、3列目)の画像と、
論文#21のFigure 6(B)の最下段のIκB-αの画像が、
同一画像であり、画像の流用(データ捏造)が疑われる。
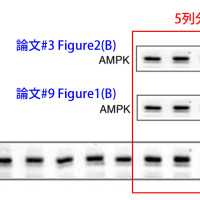
↓論文#3のFigure 2

Journal of Atherosclerosis and Thrombosis
Vol. 17 (2010) , No. 5 503-509
Copyright (c) 2010 Japan Atherosclerosis Society
(A) Cilostazol-mediated activation of AMPK in rat VSMC. VSMC were treated with colostazol (100μM) for the indicated time periods before lysis, after which samples of cell lysate were probed with antibodies specific for the phosphorylated forms of AMPK and acetyl-CoA carboxylase (ACC). (B) HUVEC treated with cilostazol (100 μM) alone or in the presence of an adenylate cyclase inhibitor SQ22536 (10 μM) or a cell-permeable cAMP analog pCTP-cAMP (100 μM). After 15 min of incubation, the cells were lysed and p-AMPK activity was analyzed. Three independent studies showed similar results.
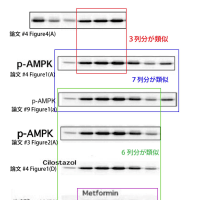
↓論文#4のFigure 1(A)(画像クリックで解像度の高い画像が表示されます。)

Figure 1
(A) Cilostazol activates AMP-activated protein kinase (AMPK) in vascular endothelial cells. Human umbilical vein endothelial cells (HUVEC) were treated with cilostazol (100 μM) for the indicated time periods before lysis, after which each cell lysate sample was probed with antibodies specific for phosphorylated forms of AMPK and acetyl-CoA carboxylase (ACC).
Hattori Y et al. Cardiovasc Res 2009;81:133-139
Copyright the European Society of Cardiology.
↓論文#4のFigure 4

Hattori Y et al. Cardiovasc Res 2009;81:133-139
Copyright the European Society of Cardiology.
Figure 4
(A) Human umbilical vein endothelial cells (HUVEC) were incubated with tumour necrosis factor alpha (TNFα) for 0–180 min. The cells were lysed and subjected to western blot analysis using anti-IκB-α and anti-phospho-IκB-α antibodies. (B) The effect of cilostazol on IκB-α degradation in HUVEC. Cells were incubated for 30 min with cilostazol (30 and 100 μM), followed by TNFα for 30 min. Cells were then lysed and subjected to western blot analysis using anti-IκBα antibody. (C) The effect of cilostazol on IKK activity in HUVEC. Cells were incubated for 30 min with cilostazol (30 and 100 μM), followed by TNFα for 15 min. Cells were then lysed and immunoprecipitated with anti-IKKα/β antibody and used for kinase assay using recombinant IκBα as a substrate. Note that equal band densities for IKKα/β and GST-IκBα were observed. Three independent studies showed similar results.
↓論文#5のFigure 3(B)

Figure 3 (b) Effect of the AMPK inhibitor compound C (CC) on
the telmisartan-mediated inhibition of TNFa-induced NF-kB activation.
Inset: Time course analysis for pAMPK by telmisartan or metformin. Data
represent the means±s.d. (n¼4). **Po0.01 compared with the control
value (TNFa only). NS, not significant between the absence and presence
of CC.
Copyright 2009 The Japanese Society of Hypertension
Hypertension Research (2009) 32, 765–769
↓論文#8のFigure 1(A)(B)

Fig. 1. (A) Fenofibrate activates AMPK in vascular endothelial cells. HUVEC were treated with 100 μmol/L fenofibrate for the indicated time periods before lysis, after which cell lysates were probed with antibodies specific for AMPK, ACC, Akt, or eNOS, or their phosphorylated forms. (B) WY14643 (100 μmol/L) activates AMPK in HUVEC.
↓論文#8のFigure 5

Fig. 5. (A) HUVEC were incubated with TNFα for 0–180 min. The cells were lysed and subjected to Western blot analysis using anti-IκB-α and anti-phospho-IκB-α antibodies. (B) The effect of fenofibrate on IκB-α degradation in HUVEC. Cells were incubated for 30 min with fenofibrate (30 and 100 μM), followed by TNFα for 30 min. Cells were then lysed and subjected to Western blot analysis using anti-IκB-α antibody. (C) The effect of fenofibrate on IKK activity in HUVEC. Cells were incubated for 30 min with metformin (30 and 100 μM), followed by TNFα for 15 min. Cells were then lysed and immunoprecipitated with anti-IKKα/β antibody and used for kinase assay using recombinant IκB-α as a substrate. Note that equal band densities for IKKα/β- and IκB-α were observed. Three independent experiments for (A) and (B) and two independent experiments for (C) were performed. Similar results were obtained in each experiment and representative photos were shown.
↓論文#9のFigure 1(A)

Figure 1 | Cilostazol activates AMP-activated protein kinase (AMPK) in
vascular endothelial cells. (a) human umbilical vein endothelial cells (HUVECs)
were treated with 100 μmol/l cilostazol for the indicated time periods before
lysis, after which cell lysates were probed with antibodies specific for AMPK,
acetyl-CoA carboxylase (ACC), Akt, or endothelial nitric oxide synthase
(eNOS), or their phosphorylated forms.
↓論文#14のFigure 3 (画像クリックで解像度の高い画像が表示されます。)

Hattori, Y. et al. Hypertension 2006;47:1183-1188
Copyright ©2006 American Heart Association
Figure 3. (A) HUVECs were incubated with TNF- for 0 to 180 minutes. The cells were lysed and subjected to Western blot analysis using anti-IkB- and anti-phospho-IkB- antibodies. (B) The effect of metformin on IB-degradation in HUVECs. Cells were incubated for 30 minutes with metformin (3 and 10 mmol/L), followed by TNF- for 30 minutes. Cells were then lysed and subjected to Western blot analysis using anti-IkB- antibody. (C) The effect of metformin on IKK activity in HUVECs. Cells were incubated for 30 minutes with metformin (3 and 10 mmol/L), followed by TNF- for 15 minutes. Cells were then lysed and immunoprecipitated with anti-IKK/ß antibody and used for kinase assay using recombinant IkB- as a substrate. Note that equal band densities for IKK/ß and IkB- were observed.
↓論文#21のFigure 6(画像をクリックすると解像度の高い画像が表示されます。)

Hattori Y et al. Cardiovasc Res 2004;63:31-40
Copyright © 2004, European Society of Cardiology
Fig. 6
(A) Effect of NOR3 and SIN-1 on degradation and phosphorylation of I B-α in rat VSMC. Cells were incubated for 45 min with various concentrations of NOR3 (1 mM) or SIN-1 (1 mM), followed by LPS (30 μg/ml) for 0–180 min. Cell were lysed and subjected to Western blot analysis using anti-I B-α antibody and anti-phospho-I B-α. (B) Effect of NOR3 and SIN-1 on IKK activity in rat VSMC. Cells were incubated for 45 min with various concentrations of NOR3 (0.25–1 mM) or SIN-1 (1 mM), followed by LPS (30 μg/ml) for 15 min. Cells were lysed and immunoprecipitated with anti-IKKα/β antibody and used for kinase assay using recombinant I B-α as substrate. Note that equal intensities of IKKα/β- and I B-α-specific bands are obtained.