再発性/難治性急性リンパ性白血病 高リスク群
MLL-AF4融合遺伝子 (+)add(4)(q31); del(11)(q13)
高白血球性白血病/貧血/血小板減少症/癌性疼痛の高リスク群
白血球過多症/癌性疼痛; 吐き気と嘔吐; 吐き気と嘔吐; 侵襲性肺アスペルギルス症/侵襲性肺真菌感染症を伴う重症免疫不全; 低タンパク血症; 急性胃粘膜病変;ヒトサイトメガロウイルス血症、電解質代謝障害、新型コロナウイルス感染、逆流性食道炎、急性移植片対宿主病(肝臓)、肝機能障害、口腔内潰瘍、菌血症(アシネトバクター・バウマニ)、皮膚軟部組織感染症(口唇潰瘍)、腎不全、消耗性重度栄養失調、末梢神経炎、化学療法後嚥下障害、低カリウム血症、侵襲性肺真菌感染症/免疫グロブリン欠乏症を伴う重度免疫不全、敗血症
2021年7月4日 COVID-19ワクチン1回目
2021年7月27日 COVID-19ワクチン2回目
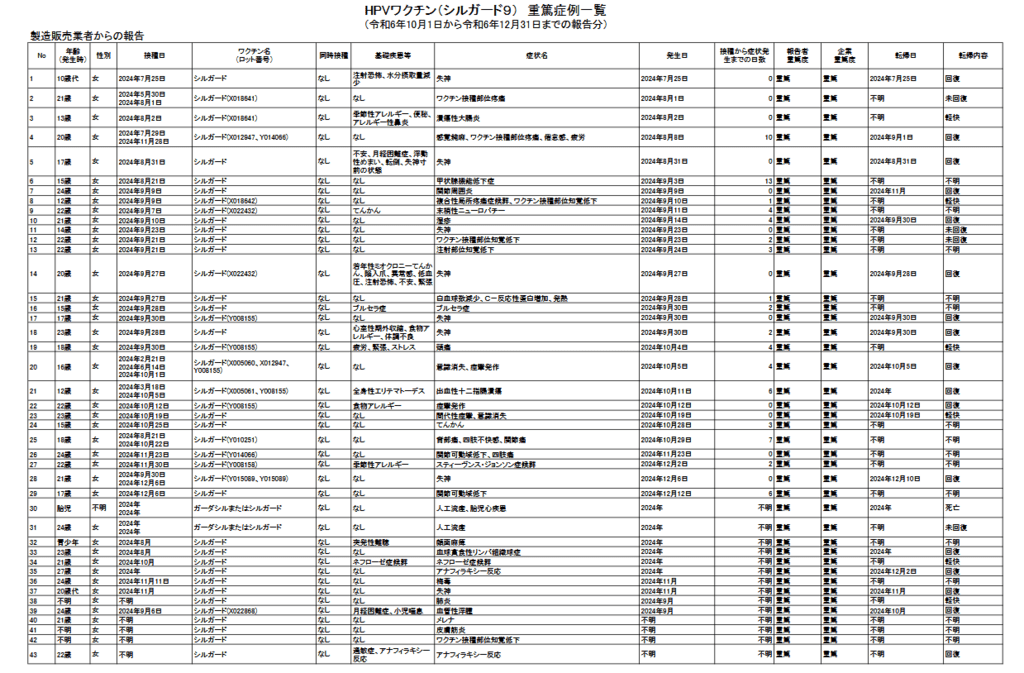
2021年12月3日 ガーダシル 1回目
2022年2月11日 ガーダシル 2回目
2022年3月12日、ガーダシル 3回目
2022年7月20日から2022年8月12日 4日以上続く腹痛と1日以上続く白血球増加」のために病院の血液内科を受診し、入院
2022年7月19日 緊急全血球算定、7月20日 白血球除去後、再度全血球算定を実施
7月21日の骨髄検査で、急性白血病(AL)の骨髄画像と一致する視野が認めらた、フローサイトメトリーでは急性Bリンパ性白血病と一致する免疫表現型が示され、B細胞性急性リンパ性白血病前駆細胞(Pro-B-ALL)が疑われた。
7月26日の染色体核型解析では、46, XX, add(4), (q31), del(11)(q13)[18]/46, XX[2]と判定され、化学療法実施
2022年12月22日から2023年2月10日 4ヶ月以上前からALLと診断され、幹細胞移植を予定しているため、入院
12月27日 Bu/Cy+ATGF前処置化学療法
1月2日 ドナー末梢血造血幹細胞119mlを輸注
2月10日 退院
2023年5月5日から27日 2日間吐き気と嘔吐、頸部と腋窩にリンパ節転移。入院後、患者は胃食道逆流症を伴う重度の吐き気と嘔吐
2023年6月11日から14日 10ヶ月以上前からALLと診断され、移植14日後再発
2023年6月28日から2023年6月30日 移植後5か月以上、6日間の頭痛、3日間の発熱
2023年7月31日から8月4日 MLL-AF4融合遺伝子陽性の急性Bリンパ芽球性白血病、同種造血幹細胞移植後の再発、重症肺炎、免疫グロブリン欠乏症、肝障害、逆流性食道炎、低タンパク血症
2023年8月5日から8月31日 咳嗽と喀痰が1週間以上続いているという理由で当院を受診し、入院
2023年10月7日から10月16日 2日間両下肢浮腫
2023年10月19日から10月21日
1. 再発・難治性ALL、高リスク群、白血球過多、全再発、2. 肺感染症を伴う重度免疫不全(細菌感染症)、3. 腹腔内感染症、4. 腫瘍崩壊症候群、5. 皮膚軟部組織感染症(口唇潰瘍)、6. 同種造血幹細胞移植後、7. 腎機能障害。 8. 吐き気と嘔吐、9. 嚥下困難、10. 逆流性食道炎、11. 肝不全、12. がん性疼痛。
2023年10月25日 非侵襲性人工呼吸器補助換気
2023年10月25日19時40分 患者の血圧と心拍数は低下し続け、患者は意識を失い、大動脈の脈動を触知できなくなった、20時27分、心電図は完全な心停止を示し、患者は臨床的に死亡宣告を受けた